Abstract
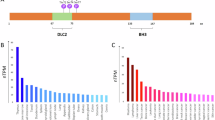
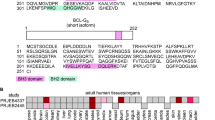
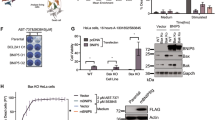
Proteins of the Bcl-2 family are critical regulators of apoptosis. Proapoptotic members, like Bax, contain three of the four Bcl-2 homology regions (BH1-3), while BH3-only proteins, like Bim, possess only the short BH3 motif. Database searches revealed Bfk, an unusual novel member of the Bcl-2 family that contains a BH2 and BH3 region but not BH1 or BH4. Bfk is thus most closely related to Bcl-GL. It lacks a C-terminal membrane anchor and is cytosolic. Enforced expression of Bfk weakly promoted apoptosis and antagonized Bcl-2's prosurvival function. Like Bcl-GL, Bfk did not bind to any Bcl-2 family members, even though its BH3 motif can mediate association with prosurvival proteins. Low amounts of Bfk were found in stomach, ovary, bone marrow and spleen, but its level in the mammary gland rose markedly during pregnancy, suggesting that Bfk may play a role in mammary development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BH:
-
Bcl-2 homology
- Bfk:
-
Bcl-2 family kin
- EST:
-
expressed sequence tag
- ORF:
-
open reading frame
- HA:
-
hemagglutinin
- MiT:
-
MSCV-IRES-Thy1.1
- MSCV:
-
mouse stem cell virus
- IRES:
-
internal ribosome entry site
- PI:
-
propidium iodide
- PCR:
-
polymerase chain reaction
- SDS:
-
sodium dodecyl sulfate
- DME:
-
Dulbecco's modified Eagle
- FCS:
-
fetal calf serum
- PAGE:
-
polyacrylamide gel electrophoresis
- FITC:
-
fluorescein isothiocyanate
- DAPI:
-
4′6-diamidino-2-phenylindole dihydrochloride
References
Cory S and Adams JM (2002) The bcl-2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2: 647–656
Huang DCS and Strasser A (2000) BH3-only proteins – essential initiators of apoptotic cell death. Cell 103: 839–842
Suzuki M, Youle RJ and Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654
Zong WX, Lindsten T, Ross AJ, MacGregor GR and Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15: 1481–1486
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T and Korsmeyer SJ (2001) BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8: 705–711
Guo B, Godzik A and Reed JC (2001) Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J. Biol. Chem. 276: 2780–2785
Kataoka T, Holler N, Micheau O, Martinon F, Tinel A, Hofmann K and Tschopp J (2001) Bcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J. Biol. Chem. 276: 19548–19554
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W and Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402
Puthalakath H, Huang DCS, O'Reilly LA, King SM and Strasser A (1999) The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3: 287–296
Negrini M, Silini E, Kozak C, Tsujimoto Y and Croce CM (1987) Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell 49: 455–463
Grillot DA, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF and Nunez G (1997) Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J. Immunol. 158: 4750–4757
Oltvai ZN, Milliman CL and Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619
Herberg JA, Phillips S, Beck S, Jones T, Sheer D, Wu JJ, Prochazka V, Barr PJ, Kiefer MC and Trowsdale J (1998) Genomic structure and domain organisation of the human Bak gene. Gene 211: 87–94
Hengartner MO and Horvitz HR (1994) C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76: 665–676
O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S and Huang DCS (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17: 384–395
Li H, Zhu H, Xu C-J and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Luo X, Budlhardjo I, Zou H, Slaughter C and Wang X (1998) Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G and Thompson CB (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608
Kumar R, Vadlamudi RK and Adam L (2000) Apoptosis in mammary gland and cancer. Endocr. Relat. Cancer 7: 257–269
Huang DCS, Cory S and Strasser A (1997) Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 14: 405–414
Hausmann G, O'Reilly LA, van Driel R, Beaumont JG, Strasser A, Adams JM and Huang DCS (2000) Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL . J. Cell Biol. 149: 623–634
Pear WS, Nolan GP, Scott ML and Baltimore D (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90: 8392–8396
O'Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DCS and Strasser A (2001) Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ. 8: 486–494
Acknowledgements
We thank Drs. D Hildeman and P Marrack for the MiT retroviral vector, C Chang and B Duscio for technical assistance, Dr. H Puthalakath for help with yeast two-hybrid analysis, Dr. L O'Reilly and J Jimenez for help with protein and antibody production and Dr. F Battye, C Tarlinton, J Chan, D Kaminaris, V Lapatis and Dr. L O'Reilly for help with flow cytometry and confocal microscopy. We are grateful to Drs. A Harris, H Puthalakath and P Bouillet for insightful discussions and critical comments on the manuscript. This work was supported by fellowships and grants from the NHMRC (Canberra), the Dr. Josef Steiner Cancer Research Foundation (Bern), the Sylvia and Charles Viertel Charitable Foundation, the Leukemia and Lymphoma Society of America and the NIH (CA80188).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Coultas, L., Pellegrini, M., Visvader, J. et al. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ 10, 185–192 (2003). https://doi.org/10.1038/sj.cdd.4401204
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401204
Keywords
This article is cited by
-
BCL-G: 20 years of research on a non-typical protein from the BCL-2 family
Cell Death & Differentiation (2023)
-
GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility
Nature Communications (2021)
-
Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-γ and TNF-α in intestinal epithelial cells
Cell Death & Disease (2020)
-
Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein
Cell Death & Disease (2014)
-
Bcl-2 family member Bcl-G is not a proapoptotic protein
Cell Death & Disease (2012)