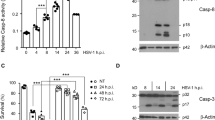
Abstract
The heat shock response and death receptor-mediated apoptosis are both key physiological determinants of cell survival. We found that exposure to a mild heat stress rapidly sensitized Jurkat and HeLa cells to Fas-mediated apoptosis. We further demonstrate that Hsp70 and the mitogen-activated protein kinases, critical molecules involved in both stress-associated and apoptotic responses, are not responsible for the sensitization. Instead, heat stress on its own induced downregulation of FLIP and promoted caspase-8 cleavage without triggering cell death, which might be the cause of the observed sensitization. Since caspase-9 and -3 were not cleaved after heat shock, caspase-8 seemed to be the initial caspase activated in the process. These findings could help understanding the regulation of death receptor signaling during stress, fever, or inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DISC:
-
death-inducing signaling complex
- DR:
-
death receptor
- ERK1/2:
-
extracellular-regulated kinase 1 and 2
- HS:
-
heat shock
- HSE:
-
heat shock element
- HSF:
-
heat shock factor
- Hsp:
-
heat shock protein
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
mitogen-activated protein kinase
References
Jolly C and Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 92: 1564–1572.
Ashkenazi A and Dixit VM (1998) Death receptors: signaling and modulation. Science 281: 1305–1308.
Ashkenazi A and Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11: 255–260.
Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S and Peter M (1998) Apoptosis signaling by death receptors. Eur. J. Biochem. 254: 439–459.
Hsu H, Shu HB, Pan MG and Goeddel DV (1996) TRADD–TRAF2 and TRADD–FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84: 299–308.
Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV and Mak TW (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279: 1954–1958.
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem. J. 326: 1–16.
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA and Dixit VM (1999) RIP3, a novel apoptosis-inducing kinase. J. Biol. Chem. 274: 16871–16875.
Stanger BZ, Leder P, Lee TH, Kim E and Seed B (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81: 513–523.
Chang HY, Nishitoh H, Yang X, Ichijo H and Baltimore D (1998) Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281: 1860–1863.
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J, Schroter M, Burns B, Mattmann C, Rimoldi D, French LE and Tschopp J (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388: 190–195.
Liu ZG, Hsu H, Goeddel DV and Karin M (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87: 565–576.
Scaffidi C, Schmitz I, Krammer PH and Peter ME (1999) The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274: 1541–1548.
Tran SE, Holmström TH, Ahonen M, Kähäri VM and Eriksson JE (2001) MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 276: 16484–16490.
Holmström TH, Tran SE, Johnson V, Ahn N, Chow SC and Eriksson J (1999) Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells. Mol. Cell. Biol. 19: 5991–6002.
Chen YR, Wang X, Templeton D, Davis RJ and Tan TH (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271: 31929–31936.
He T, Stepulak A, Holmström TH, Omary MB and Eriksson JE (2002) The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J. Biol. Chem. 277: 10767–10774.
Lindquist S and Craig EA (1988) The heat-shock proteins. Annu. Rev. Genet. 22: 631–677.
Hartl FU and Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858.
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes. Dev. 12: 3788–3796.
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11: 441–469.
Pirkkala L, Nykanen P and Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15: 1118–1131.
Holmberg CI, Tran SE, Eriksson JE and Sistonen L (2002) Multisite phosphorylation provides sophisticated regulation of transcription factors. Trends Biochem. Sci. 27: 619–627.
Kyriakis JM and Avruch J (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271: 24313–24316.
Gerner EW and Schneider MJ (1975) Induced thermal resistance in HeLa cells. Nature 256: 500–502.
Mosser DD and Martin LH (1992) Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J. Cell Physiol. 151: 561–570.
Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP and Orrenius S (2001) Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6: 49–58.
Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI and Sherman MY (1997) Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J. Biol. Chem. 272: 18033–18037.
Mosser DD, Caron AW, Bourget L, Denis-Larose C and Massie B (1997) Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17: 5317–5327.
Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD and Sherman MY (1999) Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol. Cell. Biol. 19: 2547–2555.
Saleh A, Srinivasula SM, Balkir L, Robbins PD and Alnemri ES (2000) Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2: 476–483.
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM and Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2: 469–475.
Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI and Massie B (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20: 7146–7159.
Jäättelä M, Wissing D, Kokholm K, Kallunki T and Egeblad M (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17: 6124–6134.
Gabai VL, Mabuchi K, Mosser DD and Sherman MY (2002) Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 22: 3415–3424.
Jäättelä M (1993) Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J. Immunol. 151: 4286–4294.
Jäättelä M (1999) Escaping cell death: survival proteins in cancer. Exp. Cell Res. 248: 30–43.
Liossis SN, Ding XZ, Kiang JG and Tsokos GC (1997) Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J. Immunol. 158: 5668–5675.
Xia W, Voellmy R and Spector NL (2000) Sensitization of tumor cells to fas killing through overexpression of heat-shock transcription factor 1. J. Cell. Physiol. 183: 425–431.
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252.
Arrigo AP (1998) Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 379: 19–26.
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288: 870–874.
Yang D, Tournier C, Wysk M, Lu HT, Xu J, Davis RJ and Flavell RA (1997) Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc. Natl. Acad. Sci. USA 94: 3004–3009.
Reap EA, Roof K, Maynor K, Borrero M, Booker J and Cohen PL (1997) Radiation and stress-induced apoptosis: a role for Fas/Fas ligand interactions. Proc. Natl. Acad. Sci. USA 94: 5750–5755.
Faris M, Kokot N, Latinis K, Kasibhatla S, Green DR, Koretzky GA and Nel A (1998) The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J. Immunol. 160: 134–144.
Carmody RJ and Cotter TG (2000) Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 7: 282–291.
Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M and Jäättelä M (2001) Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153: 999–1010.
Leist M and Jäättelä M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2: 589–598.
Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N and Kroemer G (2002) Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 84: 215–222.
Mathiasen IS and Jäättelä M (2002) Triggering caspase-independent cell death to combat cancer. Trends Mol. Med. 8: 212–220.
Krueger A, Schmitz I, Baumann S, Krammer PH and Kirchhoff S (2001) Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276: 20633–20640.
Kataoka T, Ito M, Budd RC, Tschopp J and Nagai K (2002) Expression level of c-FLIP versus Fas determines susceptibility to Fas ligand-induced cell death in murine thymoma EL-4 cells. Exp. Cell Res. 273: 256–264.
Gross A, McDonnell J and Korsmeyer S (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13: 1899–1911.
Creagh EM, Carmody RJ and Cotter TG (2000) Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp. Cell Res. 257: 58–66.
Tibbles LA and Woodgett JR (1999) The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 55: 1230–1254.
Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM and Hood L (1997) MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc. Natl. Acad. Sci. USA 94: 11333–11338.
Shu HB, Halpin DR and Goeddel DV (1997) Casper is a FADD- and caspase-related inducer of apoptosis. Immunity 6: 751–763.
Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME and Yang X (2002) c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 21: 3704–3714.
Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C and Grutter MG (2002) The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 277: 45162–45171.
Hasday JD and Singh IS (2000) Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones 5: 471–480.
Hobohm U (2001) Fever and cancer in perspective. Cancer Immunol. Immunother. 50: 391–396.
Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R and Riess H (2002) The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 43: 33–56.
Medema JP, de Jong J, van Hall T, Melief CJ and Offringa R (1999) Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J. Exp. Med. 190: 1033–1038.
Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P and Grandien A (1999) The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190: 1025–1032.
Thomas RK, Kallenborn A, Wickenhauser C, Schultze JL, Draube A, Vockerodt M, Re D, Diehl V and Wolf J (2002) Constitutive expression of c-FLIP in Hodgkin and Reed-Sternberg cells. Am. J. Pathol. 160: 1521–1528.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W and Bujard H (1995) Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769.
Caron AW, Massie B and Mosser DD (2000) Use of a micromanipulator for high-efficiency cloning of cells co-expressing fluorescent proteins. Methods Cell Sci. 22: 137–145.
Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM and Zon LI (1994) Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372: 794–798.
Scaffidi C, Medema JP, Krammer PH and Peter ME (1997) FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol. Chem. 272: 26953–26958.
Mosser DD, Theodorakis NG and Morimoto RI (1988) Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol. Cell. Biol. 8: 4736–4744.
Acknowledgements
We thank Peter Krammer (German Cancer Research Center, Heidelberg, Germany) for caspase-8 and FLIP antibodies, Jürg Tschopp (Institute of Biochemistry, University of Lausanne, Switzerland) for Fas ligand, and John Kyriakis (Diabetes Research Laboratory, Massachusetts General Hospital, Charlestown, MA, USA) for the SEK1 plasmid. We also thank the other members of our laboratories for technical help and critical comments on the manuscript. Financial support from the Academy of Finland, Finnish Cancer Organizations, Sigrid Jusélius Foundation, Erna and Victor Hasselblad Foundation, Nordic Academy for Advanced Study (NorFA), Magnus Ehrnrooth Foundation, Åbo Akademi University, and University of Turku is gratefully acknowledged. SEFT was supported by the Turku Graduate School of Biomedical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by E Alnemri
Rights and permissions
About this article
Cite this article
Tran, S., Meinander, A., Holmström, T. et al. Heat stress downregulates FLIP and sensitizes cells to Fas receptor-mediated apoptosis. Cell Death Differ 10, 1137–1147 (2003). https://doi.org/10.1038/sj.cdd.4401278
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401278
Keywords
This article is cited by
-
In vitro effects of 5-Hydroxy-L-tryptophan supplementation on primary bovine mammary epithelial cell gene expression under thermoneutral or heat shock conditions
Scientific Reports (2022)
-
MicroRNA-216b inhibits heat stress-induced cell apoptosis by targeting Fas in bovine mammary epithelial cells
Cell Stress and Chaperones (2018)
-
Roles of heat shock factor 1 beyond the heat shock response
Cellular and Molecular Life Sciences (2018)
-
Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion
Cell Death & Disease (2015)
-
Hyperthermia: an effective strategy to induce apoptosis in cancer cells
Apoptosis (2015)