Abstract
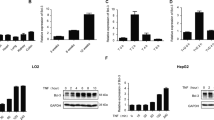
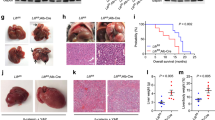
The liver is particularly susceptible to Fas-mediated cytotoxicity. Mice given an adequate parenteral dose of agonistic anti-Fas antibody (aFas) or of FasL are known to develop a devastating liver injury and to die in a few hours. The present work shows that mice lacking TNFR1 and TNFR2 (R−) both survive a single dose of aFas, otherwise rapidly lethal, and develop a mild form of hepatic damage, compared to the much more severe liver injury that in a few hours strikes wild-type mice (R+), eventually involving increased activity of proteases of different families (caspase 3-, 8-, and 9-like, calpains, cathepsin B). Neither the overall tissue levels of Fas and FasL nor Fas expression at the hepatocyte surface are altered in the liver of R− animals. The DNA-binding activity of the NF-κB transcription factor is enhanced after aFas treatment, but much more markedly in R− than in R+ mice. Bcl2, while unchanged in untreated animals, is markedly upregulated in R− but not in R+ mice challenged with aFas. The requirement of a normal TNFR1/TNFR2 phenotype for full deployment of the general and liver-specific aFas toxicity in mice most likely implies that treatment with aFas in some way results in activation of the TNFα-TNFRs system and that this activation synergizes with Fas-mediated signals in causing the fulminant liver injury and the animal death. The precise cellular and molecular details underlying this interplay between Fas- and TNFRs-mediated signaling systems in the general and liver-specific aFas toxicity largely remain to be clarified.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- aFas:
-
agonistic anti-Fas antibody
- R+:
-
normal mice
- R−:
-
mice knocked out for TNF-R1 and TNF-R2
References
Desbarats J and Newell MK (2000) Fas engagement accelerates liver regeneration after partial hepatectomy. Nat. Med. 6: 920–923.
Tran SE, Holmstrom TH, Ahonen M, Kahari VM and Eriksson JE (2001) MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 276: 16484–16490.
Budd RC (2002) Death receptors couple to both cell proliferation and apoptosis. J. Clin. Invest. 109: 437–441.
Nagata S and Golstein P (1995) The Fas death factor. Science 267: 1449–1456.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17: 1675–1687.
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH and Peter ME (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274: 22532–22538.
Pinkoski MJ, Brunner T, Green DR and Lin T (2000) Fas and Fas ligand in gut and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 278: G354–G366.
Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH and Runkel L (1995) Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 182: 1223–1230.
Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H and Kamada T (1994) Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology 19: 1354–1359.
Mochizuki K, Hayashi N, Hiramatsu N, Katayama K, Kawanishi Y, Kasahara A, Fusamoto H and Kamada T (1996) Fas antigen expression in liver tissues of patients with chronic hepatitis B. J. Hepatol. 24: 1–7.
Kondo T, Suda T, Fukuyama H, Adachi M and Nagata S (1997) Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3: 409–413.
Seishima M, Takemura M, Saito K, Ando K and Noma A (1997) Increased serum soluble Fas (sFas) concentrations in HCV-positive patients with liver cirrhosis. J. Hepatol. 27: 424–425.
Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J and Nagata S (1994) Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp. Cell Res. 215: 332–337.
Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T and Nagata S (1993) Lethal effect of the anti-Fas antibody in mice. Nature 364: 806–809.
Ledgerwood EC, Pober JS and Bradley JR (1999) Recent advances in the molecular basis of TNF signal transduction. Lab. Invest. 79: 1041–1050.
Rolfe M, James NH and Roberts RA (1997) Tumour necrosis factor alpha (TNF alpha) suppresses apoptosis and induces DNA synthesis in rodent hepatocytes: a mediator of the hepatocarcinogenicity of peroxisome proliferators? Carcinogenesis 18: 2277–2278.
Xu Y, Jones BE, Neufeld DS and Czaja MJ (1998) Glutathione modulates rat and mouse hepatocyte sensitivity to tumor necrosis factor toxicity. Gastroenterology 115: 1229–1237.
Michalopoulos GK and DeFrances MC (1997) Liver regeneration. Science 276: 60–66.
Takehara T, Hayashi N, Mita E, Kanto T, Tatsumi T, Sasaki Y, Kasahara A and Hori M (1998) Delayed Fas-mediated hepatocyte apoptosis during liver regeneration in mice: hepatoprotective role of TNF alpha. Hepatology 27: 1643–1651.
Yamada Y and Fausto N (1998) Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am. J. Pathol. 152: 1577–1589.
Rose ML, Bradford BU, Germolec DR, Lin M, Tsukamoto H and Thurman RG (2001) Gadolinium chloride-induced hepatocyte proliferation is prevented by antibodies to tumor necrosis factor alpha. Toxicol. Appl. Pharmacol. 170: 39–45.
Tiegs G, Wolter M and Wendel A (1989) Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 38: 627–631.
Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H and Fujiwara H (1994) T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 179: 1529–1537.
Bradham CA, Plumpe J, Manns MP, Brenner DA and Trautwein C (1998) Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am. J. Physiol. 275: G387–G392.
Plumpe J, Malek NP, Bock CT, Rakemann T, Manns MP and Trautwein C (2000) NF-kappaB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 278: G173–G183.
Jones BE, Lo CR, Liu H, Pradhan Z, Garcia L, Srinivasan A, Valentino KL and Czaja MJ (2000) Role of caspases and NF-kappaB signaling in hydrogen peroxide- and superoxide-induced hepatocyte apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 278: G693–G699.
Czaja MJ (2001) TNF toxicity – death from caspase or cathepsin, that is the question. Hepatology 34: 844–846.
Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG and Wendel A (1995) Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 146: 1220–1234.
Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H and Kunzendorf U (1997) Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 3: 1124–1128.
Bulfone-Paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R and Krause H (1999) Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 13: 1575–1585.
Wanner GA, Mica L, Wanner-Schmid E, Kolb SA, Hentze H, Trentz O and Ertel W (1999) Inhibition of caspase activity prevents CD95-mediated hepatic microvascular perfusion failure and restores Kupffer cell clearance capacity. FASEB J. 13: 1239–1248.
Hoglen NC, Hirakawa BP, Fisher CD, Weeks S, Srinivasan A, Wong AM, Valentino KL, Tomaselli KJ, Bai X, Karanewsky DS and Contreras PC (2001) Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J. Pharmacol. Exp. Ther. 297: 811–818.
Ruiz-Vela A, Gonzalez de Buitrago G and Martinez-A C (1999) Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 18: 4988–4998.
Chen M, He H, Zhan S, Krajewski S, Reed JC and Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J. Biol. Chem. 276: 30724–30728.
Varghese J, Radhika G and Sarin A (2001) The role of calpain in caspase activation during etoposide induced apoptosis in T cells. Eur. J. Immunol. 31: 2035–2041.
Ding WX, Shen HM and Ong CN (2002) Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem. Biophys. Res. Commun. 291: 321–331.
Deiss LP, Galinka H, Berissi H, Cohen O and Kimchi A (1996) Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15: 3861–3870.
Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M and Jaattela M (2001) Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153: 999–1010.
Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH and Gores GJ (2000) Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106: 1127–1137.
Spanaus KS, Schlapbach R and Fontana A (1998) TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xL. Eur. J. Immunol. 28: 4398–4408.
Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V and Kahn A (1996) Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat. Med. 2: 80–86.
Rodriguez I, Matsuura K, Khatib K, Reed JC, Nagata S and Vassalli P (1996) A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J. Exp. Med. 183: 1031–1036.
Mukherjee D, Kaestner KH, Kovalovich KK and Greenbaum LE (2001) Fas-induced apoptosis in mouse hepatocytes is dependent on C/EBPbeta. Hepatology 33: 1166–1172.
Chung JY, Park YC, Ye H and Wu H (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115: 679–688.
Matsuki Y, Li L, Hsu HC, Yang PA, Zheng R, Edwards III CK, Chaudry IH, Zhang HG and Mountz JD (2002) Soluble Fas gene therapy protects against Fas-mediated apoptosis of hepatocytes but not the lethal effects of Fas-induced TNF-alpha production by Kupffer cells. Cell Death Differ. 9: 626–635.
Rai RM, Zhang JX, Clemens MG and Diehl AM (1996) Gadolinium chloride alters the acinar distribution of phagocytosis and balance between pro- and anti-inflammatory cytokines. Shock 6: 243–247.
Rai RM, Loffreda S, Karp CL, Yang SQ, Lin HZ and Diehl AM (1997) Kupffer cell depletion abolishes induction of interleukin-10 and permits sustained overexpression of tumor necrosis factor alpha messenger RNA in the regenerating rat liver. Hepatology 25: 889–895.
Yonehara S, Ishii A and Yonehara M (1989) A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J. Exp. Med. 169: 1747–1756.
Teh HS, Seebaran A and Teh SJ (2000) TNF receptor 2-deficient CD8 T cells are resistant to Fas/Fas ligand-induced cell death. J. Immunol. 165: 4814–4821.
Varfolomeev EE, Boldin MP, Goncharov TM and Wallach D (1996) A potential mechanism of ‘cross-talk’ between the p55 tumor necrosis factor receptor and Fas/APO1: proteins binding to the death domains of the two receptors also bind to each other. J. Exp. Med. 183: 1271–1275.
Bang S, Jeong EJ, Kim IK, Jung YK and Kim KS (2000) Fas- and tumor necrosis factor-mediated apoptosis uses the same binding surface of FADD to trigger signal transduction. A typical model for convergent signal transduction. J. Biol. Chem. 275: 36217–36222.
Kim BC, Kim HT, Mamura M, Ambudkar IS, Choi KS and Kim SJ (2002) Tumor necrosis factor induces apoptosis in hepatoma cells by increasing Ca(2+) release from the endoplasmic reticulum and suppressing Bcl-2 expression. J. Biol. Chem. 277: 31381–31389.
Kalb A, Bluethmann H, Moore MW and Lesslauer W (1996) Tumor necrosis factor receptors (Tnfr) in mouse fibroblasts deficient in Tnfr1 or Tnfr2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J. Biol. Chem. 271: 28097–28104.
Kaufmann SH (1989) Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 49: 5870–5878.
Lowry OH, Rosebrough NJ, Farr AL and Randall (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Acknowledgements
This work has been supported by the Ministero per l'Istruzione, l'Università e la Ricerca (MIUR, Roma; Italy-Spain Integrated Actions). P Aoki is recipient of a fellowship from the Fundacion Antorchas, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G. Ciliberto
Rights and permissions
About this article
Cite this article
Costelli, P., Aoki, P., Zingaro, B. et al. Mice lacking TNFα receptors 1 and 2 are resistant to death and fulminant liver injury induced by agonistic anti-Fas antibody. Cell Death Differ 10, 997–1004 (2003). https://doi.org/10.1038/sj.cdd.4401281
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401281
Keywords
This article is cited by
-
Disruption of the FasL/Fas axis protects against inflammation-derived tumorigenesis in chronic liver disease
Cell Death & Disease (2019)
-
TNFα sensitizes hepatocytes to FasL-induced apoptosis by NFκB-mediated Fas upregulation
Cell Death & Disease (2018)
-
Construction of mTNFR1shRNA plasmid and its biological effects on MHV-3 induced fulminant hepatitis in BALB/cJ mice
Virologica Sinica (2010)
-
Protective effects of HFE7A, mouse anti-human/mouse Fas monoclonal antibody against acute and lethal hepatic injury induced by Jo2
Cytotechnology (2010)
-
Immune cell-mediated liver injury
Seminars in Immunopathology (2009)