Abstract
The CD95 ligand is involved as a death factor in the regulation of activation-induced cell death, establishment of immune privilege and tumor cell survival. In addition, CD95L may serve as a costimulatory molecule for T-cell activation. Alterations in expression or shedding of membrane and soluble CD95L are associated with numerous diseases, and underscore the pathophysiological relevance of the CD95/CD95L system. In most cases, the causal link between altered CD95L expression and pathophysiology is unknown. Given the potency of the molecule to regulate death and survival of many different cell types, the control of CD95L production, transport, storage, shedding and inactivation is of tremendous biological and clinical interest. This review summarizes the current knowledge, hypotheses and controversies about CD95L as a multifunctional ligand and receptor. It considers the different roles of membrane and soluble forms of CD95L and the complex networks of intracellular dynamics of protein trafficking, as well as the potential bidirectional signal transduction capacity of CD95L, with a focus on molecular interactions that have been worked out over the past years.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- aa:
-
amino acids
- ADAM:
-
a-disintegrin-and metalloproteinase
- AICD:
-
activation-induced cell death
- CD95L:
-
CD95 ligand
- FBP:
-
forming-binding protein
- GST:
-
glutathione-S-transferase
- MALDI-TOF:
-
matrix-assisted laser disorption ionization time of flight
- (M)MP:
-
(matrix)metalloproteinase
- PACSIN:
-
protein kinase C and casein kinase substrate in neurons
- PRD:
-
proline-rich domain
- SH:
-
Src homology
- TCR:
-
T-cell receptor
- TIL:
-
tumor-infiltrating lymphocytes
- TNF-R:
-
tumor necrosis factor receptor
References
Suda T, Takahashi T, Golstein P and Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75: 1169–1178
Green DR, Droin N and Pinkoski M (2003) Activation-induced cell death in T cells. Immunol. Rev. 193: 70–81
Eischen CM, Schilling JD, Lynch DH, Krammer PH and Leibson PJ (1996) Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J. Immunol. 156: 2693–2699
Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC and Liles WC (1997) Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J. Immunol. 159: 1594–1598
De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U and Peschle C (1999) Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature 401: 489–493
Kaplan HJ, Leibole MA, Tezel T and Ferguson TA (1999) Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat. Med. 5: 292–297
Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN and Owen-Schaub LB (1999) Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science 285: 898–900
Pinkoski MJ and Green DR (1999) Fas ligand, death gene. Cell Death Differ. 6: 1174–1181
Restifo NP (2000) Not so Fas: re-evaluating the mechanisms of immune privilege and tumor escape. Nat. Med. 6: 493–495
O'Connell J, Houston A, Bennett MW, O'Sullivan GC and Shanahan F (2001) Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat. Med. 7: 271–274
Ferguson TA, Green DR and Griffith TS (2002) Cell death and immune privilege. Int. Rev. Immunol. 21: 153–172
Nakajima M, Nakajima A, Kayagaki N, Honda M, Yagita H and Okumura K (1997) Expression of Fas ligand and its receptor in cutaneous lupus: implication in tissue injury. Clin. Immunol. Immunopathol. 83: 223–229
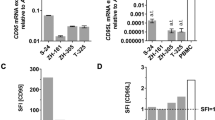
Kavurma MM and Khachigian LM (2003) Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ. 10: 36–44
Nagata S (1999) Fas ligand-induced apoptosis. Annu. Rev. Genet. 33: 29–55
Krammer PH (1999) CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71: 163–210
Peter ME and Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10: 26–35
Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D and Ashkenazi A (1998) Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396: 699–703
Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, Bahr M, Ohgaki H, Ashkenazi A and Weller M (2001) Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 61: 2759–2765
Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, Engel J, Schneider P and Tschopp J (2003) Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol. Cell Biol. 23: 1428–1440
Ayroldi E, D'Adamio F, Zollo O, Agostini M, Moraca R, Cannarile L, Migliorati G, Delfino DV and Riccardi C (1999) Cloning and expression of a short Fas ligand: a new alternatively spliced product of the mouse Fas ligand gene. Blood 94: 3456–3467
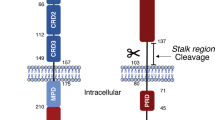
Tanaka M, Itai T, Adachi M and Nagata S (1998) Downregulation of Fas ligand by shedding. Nat. Med. 4: 31–36
Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A and Tschopp J (1998) Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187: 1205–1213
Vargo-Gogola T, Crawford HC, Fingleton B and Matrisian LM (2002) Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch. Biochem. Biophys. 408: 155–161
Powell WC, Fingleton B, Wilson CL, Boothby M and Matrisian LM (1999) The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9: 1441–1447
Crawford HC, Scoggins CR, Washington MK, Matrisian LM and Leach SD (2002) Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J. Clin. Invest. 109: 1437–1444
Strand S, Vollmer P, van den Abeelen L, Kuball J, Heid H, Theobald M, Galle PR and Strand D (2002) Cleavage of CD95 (APO-1, Fas) by matrilysin (MMP-7) induces apoptoaia resistance in tumor cells. Immunobiology 206: 247
Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond AH and Nagata S (1996) Fas ligand in human serum. Nat. Med. 2: 317–322
Bahr GM, Capron A, Dewulf J, Nagata S, Tanaka M, Bourez JM and Mouton Y (1997) Elevated serum level of Fas ligand correlates with the asymptomatic stage of human immunodeficiency virus infection. Blood 90: 896–898
Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG and Nabel GJ (2001) Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat. Immunol. 2: 333–337
Seino K, Iwabuchi K, Kayagaki N, Miyata R, Nagaoka I, Matsuzawa A, Fukao K, Yagita H and Okumura K (1998) Chemotactic activity of soluble Fas ligand against phagocytes. J. Immunol. 161: 4484–4488
Ottonello L, Tortolina G, Amelotti M and Dallegri F (1999) Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J. Immunol. 162: 3601–3606
Igney FH and Krammer PH (2002) Immune escape of tumors: apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 71: 907–920
Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G and Fais S (2002) Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 195: 1303–1316
Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S and Parmiani G (2002) Immunity to cancer: attack and escape in T lymphocyte–tumor cell interaction. Immunol. Rev. 188: 97–113
Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J and Tschopp J (1996) Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 274: 1363–1366
Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T and Nagata S (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76: 969–976
Rieux-Laucat F, Le Deist F and Fischer A (2003) Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death Differ. 10: 124–133
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J and French LE (1998) Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 282: 490–493
Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, Weller M and Gulbins E (2000) CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290: 527–530
Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H and Schonrich G (2001) Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15: 997–1009
Matiba B, Mariani SM and Krammer PH (1997) The CD95 system and the death of a lymphocyte. Semin. Immunol. 9: 59–68
Kishimoto H and Sprent J (2000) The thymus and negative selection. Immunol. Res. 21: 315–323
Sprent J and Kishimoto H (2001) The thymus and central tolerance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356: 609–616
Singer GG and Abbas AK (1994) The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1: 365–371
Janssen O, Stocker A, Sanzenbacher R, Oberg HH, Siddiqi MA and Kabelitz D (2000) Differential regulation of activation-induced cell death in individual human T cell clones. Int. Arch. Allergy Immunol. 121: 183–193
Janssen O, Sanzenbacher R and Kabelitz D (2000) Regulation of activation-induced cell death of mature T-lymphocyte populations. Cell Tissue Res. 301: 85–99
Blott EJ and Griffiths GM (2002) Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3: 122–131
Stinchcombe JC, Bossi G, Booth S and Griffiths GM (2001) The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15: 751–761
Suzuki I and Fink PJ (1998) Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J. Exp. Med. 187: 123–128
Suzuki I and Fink PJ (2000) The dual functions of fas ligand in the regulation of peripheral CD8+ and CD4+ T cells. Proc. Natl. Acad. Sci. USA 97: 1707–1712
Desbarats J, Duke RC and Newell MK (1998) Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat. Med. 4: 1377–1382
Suzuki I, Martin S, Boursalian TE, Beers C and Fink PJ (2000) Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J. Immunol. 165: 5537–5543
Locksley RM, Killeen N and Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104: 487–501
Wenzel J, Sanzenbacher R, Ghadimi M, Lewitzky M, Zhou Q, Kaplan DR, Kabelitz D, Feller SM and Janssen O (2001) Multiple interactions of the cytosolic polyproline region of the CD95 ligand: hints for the reverse signal transduction capacity of a death factor. FEBS Lett. 509: 255–262
Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U and Pfeffer K (2002) Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J. Exp. Med. 195: 1613–1624
Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H and Ware CF (2001) Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J. Immunol. 167: 6330–6337
Morel Y, Truneh A, Sweet RW, Olive D and Costello RT (2001) The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J. Immunol. 167: 2479–2486
Chou AH, Tsai HF, Lin LL, Hsieh SL, Hsu PI and Hsu PN (2001) Enhanced proliferation and increased IFN-gamma production in T cells by signal transduced through TNF-related apoptosis-inducing ligand. J. Immunol. 167: 1347–1352
Watts AD, Hunt NH, Wanigasekara Y, Bloomfield G, Wallach D, Roufogalis BD and Chaudhri G (1999) A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling’. EMBO J. 18: 2119–2126
Hane M, Lowin B, Peitsch M, Becker K and Tschopp J (1995) Interaction of peptides derived from the Fas ligand with the Fyn-SH3 domain. FEBS Lett. 373: 265–268
Ghadimi MP, Sanzenbacher R, Thiede B, Wenzel J, Jing Q, Plomann M, Borkhardt A, Kabelitz D and Janssen O (2002) Identification of interaction partners of the cytosolic polyproline region of CD95 ligand (CD178). FEBS Lett. 519: 50–58
Leo A and Schraven B (2001) Adapters in lymphocyte signalling. Curr. Opin. Immunol. 13: 307–316
Lussier G and Larose L (1997) A casein kinase I activity is constitutively associated with Nck. J. Biol. Chem. 272: 2688–2694
Tanaka K (2000) Formin family proteins in cytoskeletal control. Biochem. Biophys. Res. Commun. 267: 479–481
Fuchs U, Rehkamp G, Haas OA, Slany R, Konig M, Bojesen S, Bohle RM, Damm-Welk C, Ludwig WD, Harbott J and Borkhardt A (2001) The human formin-binding protein 17 (FBP17) interacts with sorting nexin, SNX2, and is an MLL-fusion partner in acute myelogeneous leukemia. Proc. Natl. Acad. Sci. USA 98: 8756–8761
Worby CA and Dixon JE (2002) Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 3: 919–931
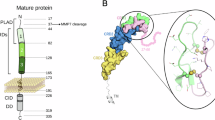
Blott EJ, Bossi G, Clark R, Zvelebil M and Griffiths GM (2001) Fas ligand is targeted to secretory lysosomes via a proline-rich domain in its cytoplasmic tail. J. Cell Sci. 114: 2405–2416
Gil D, Schamel WW, Montoya M, Sanchez-Madrid F and Alarcon B (2002) Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109: 901–912
Bossi G, Stinchcombe JC, Page LJ and Griffiths GM (2000) Sorting out the multiple roles of Fas ligand. Eur. J. Cell Biol. 79: 539–543
Russell JH and Ley TJ (2002) Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20: 323–370
Bossi G and Griffiths GM (1999) Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 5: 90–96
Acknowledgements
#OJ is supported by grants from the Deutsche Forschungsgemeinschaft (SFB 415) and the Faculty of Medicine, University of Kiel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by T Ferguson
Rights and permissions
About this article
Cite this article
Janssen, O., Qian, J., Linkermann, A. et al. CD95 ligand - death factor and costimulatory molecule?. Cell Death Differ 10, 1215–1225 (2003). https://doi.org/10.1038/sj.cdd.4401305
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401305
Keywords
This article is cited by
-
Tau Pathology Triggered by Spinal Cord Injury Can Play a Critical Role in the Neurotrauma Development
Molecular Neurobiology (2020)
-
Impairment of Fas-ligand–caveolin-1 interaction inhibits Fas-ligand translocation to rafts and Fas-ligand-induced cell death
Cell Death & Disease (2018)
-
Neutralization of CD95 ligand protects the liver against ischemia-reperfusion injury and prevents acute liver failure
Cell Death & Disease (2018)
-
The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood
Spinal Cord (2017)
-
Role of Bcl-2 family proteins and caspases in the regulation of apoptosis
Molecular and Cellular Biochemistry (2011)