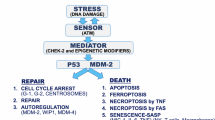
Abstract
While it is well accepted that p53 plays a role in apoptosis, less is known as to its involvement in cell differentiation. Here we show that wild-type p53 facilitates IL-6-dependent macrophage differentiation. Treatment of M1/2 cells expressing the temperature-sensitive p53 143 (Val to Ala) mutant, at the wild-type conformation, facilitated the appearance of mature macrophages that exhibited phagocytic activity. Enhancement of differentiation by the p53 143 (Val to Ala) in the wild-type conformation was coupled with the inhibition of apoptosis induction by this protein. In agreement with previous studies, we found that p53 levels were reduced during p53-dependent macrophage differentiation. This occurred when p53 levels before IL-6 stimuli were high. Interestingly, the p53 143 (Val to Ala) protein, at the mutant conformation, enhanced macrophage differentiation, as did the wild-type conformation, whereas the p53 273 (Arg to His) core mutant exerted an inhibitory effect on this pathway. The transcription-deficient p53 molecules, p53 (22–23) and p53 22,23,143, could not induce p53-dependent differentiation. Moreover, the p53 (22–23) protein inhibited the p53-independent differentiation pathway. Interestingly, the p53 (22–23) protein not only blocked IL-6-mediated differentiation, but also induced significant apoptotic cell death, upon IL-6 stimulation. Taken together, our data show that wild-type p53 enhances macrophage differentiation, while various p53 mutant types exert different effects on this differentiation pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AO:
-
Acridine orange
- DMEM:
-
Dulbecco's modified Eagle's medium
- HBSS:
-
Hank's balanced salt solution
- HRP:
-
Horseradish peroxidase
- IL-6:
-
interleukin-6
- TRAP:
-
telomeric repeat amplification protocol
References
Metcalf D (1993) Hematopoietic regulators: redundancy or subtlety? Blood 82: 3515–3523
Metcalf D (1991) Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science 254: 529–533
Friedman AD (2002) Transcriptional regulation of granulocyte and monocyte development. Oncogene 21: 3377–3390
Schwartz D and Rotter V (1998) p53-dependent cell cycle control: response to genotoxic stress. Semin. Cancer Biol. 8: 325–336
Sionov RV and Haupt Y (1999) The cellular response to p53: the decision between life and death. Oncogene 18: 6145–6157
Ko JL and Prives C (1996) p53: puzzle and paradigm. Genes Dev. 10: 1054–1072
El-Deiry WS (1998) Regulation of p53 downstream genes. Semin. Cancer Biol. 8: 345–357
Gottlieb TM and Oren M (1998) p53 and apoptosis. Semin. Cancer Biol. 8: 359–368
Itahana K, Dimri G and Campisi J (2001) Regulation of cellular senescence by p53. Eur. J. Biochem. 268: 2784–2791
Janus F, Albrechtsen N, Dornreiter I, Wiesmuller L, Grosse F and Deppert W (1999) The dual role model for p53 in maintaining genomic integrity. Cell Mol. Life Sci. 55: 12–27
Tlsty TD (1997) Genomic instability and its role in neoplasia. Curr Top Microbiol Immunol. 221: 37–46
Wahl GM, Linke SP, Paulson TG and Huang LC (1997) Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv. 29: 183–219
Almog N and Rotter V (1997) Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta 1333: F1–F27
Donehower LA, Harvey M, Slagle BT, McArthur MJ, Montgomery CA, Butel JS and Bradly A (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 356: 215–221
Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT and Jacks T (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat. Genet. 10: 175–180
Armstrong JF, Kaufman MH, Harrison DJ and Clarke AR (1995) High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 5: 931–936
Choi J and Donehower LA (1999) p53 in embryonic development: maintaining a fine balance. Cell Mol. Life Sci. 55: 38–47
Shaulsky G, Goldfinger N, Peled A, Rotter and V (1991) Involvement of wild type p53 in pre-B cell differentiation in vitro. Proc. Natl. Acad. Sci. USA 88: 8982–8986
Shaulsky G, Goldfinger N and Rotter V (1991) Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res. 51: 5232–5237
Aloni-Grinstein R, Schwartz D and Rotter V (1995) Accumulation of wild-type p53 protein upon gamma-irradiation induces a G2 arrest-dependent immunoglobulin kappa light chain gene expression. EMBO J 14: 1392–1401
Soddu S, Blandino G, Citro G, Scardigli R, Piaggio G, Ferber A, Calabretta B and Sacchi A (1994) Wild-type p53 gene expression induces granulocytic differentiation of HL-60 cells. Blood 83: 2230–2237
Banerjee D, Lenz H, Schnieders B, Manno JD, Ju FJ, Spears CP, Hochhauser D, Danenberg K, Danenberg P and Bertino RJ (1995) Transfection of wild-type but not mutant p53 induces early monocytic differentiation in HL60 cells and increases their sensitivity to stress. Cell Growth Differ. 6: 1405–1413
Feinstein E, Gale PB, Reed J and Cannai E (1992) Expression of the normal p53 gene induces differentiation of K562 cells. Oncogene 7: 1853–1857
Ehinger M, Nilsson E, Persson A, Olsson I and Gullberg U (1995) Involvement of the tumor suppressor gene p53 in tumor necrosis factor-induced differentiation of the leukemic cell line K562. Cell Growth Differ. 6: 9–17
Soddu S, Blandino G, Scardigli R, Coen S, Marchetti A, Rizzo MG, Bossi G, Cimino L, Crescenzi M and Sacchi A (1996) Interference with p53 protein inhibits hematopoietic and muscle differentiation. J. Cell Biol. 134: 1–12
Johnson P, Chung S and Benchimol S (1993) Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobulin production and is sensitive to erythropoitin. Mol. Cell. Biol. 13: 1456–1463
Kastan MB, Radin AI, Kuerbitz SJ, Onyekwere O, Wolkow CA, Civin CI, Stone KD, Woo T, Ravindranath Y and Craig RW (1991) Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res. 51: 4279–4286
Weinberg CW, Azzoli GC, Chapman K, Levine JA and Yuspa HS (1995) p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene 10: 2271–2279
Lutzker SG and Levine AJ (1996) A functionally inactive p53 protein in teratocacinoma cells is activated by either DNA damage or cellular differentiation. Nat. Med. 2: 804–810
Sabapathy K, Klemm M, Jaenisch R and Wagner EF (1997) Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J. 16: 6217–6229
Aloni-Grinstein R, Zan-Bar I, Alboum I, Goldfinger N and Rotter V (1993) Wild type p53 functions as a control protein in the differentiation pathway of the B-cell lineage. Oncogene 8: 3297–3305
Weintraub H, Hauschka S and Tapscott SJ (1991) The MCK enhancer contains a p53 responsive elements. Proc. Natl. Acad. Sci. USA 88: 4570–4571
Saifudeen Z, Dipp S and El-Dahr SS (2002) A role for p53 in terminal epithelial cell differentiation. J. Clin. Invest. 109: 1021–1030
Hussain SP and Harris CC (1998) Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 58: 4023–4037
Feinstein E, Cimino G, Gale RP, Alimena G, Berthier R, Kishi K, Goldman J, Zaccaria A, Berrebi A and E C (1991) p53 in chronic myelogenous leukemia in acute phase. Proc. Natl. Acad. Sci. USA 88: 6293–6297
Fagin JA (1995) Tumor supresor genes in human thyroid neoplasma: p53 mutations are associated with undifferentiated thyroid cancers. J. Endocrinol. Invest. 18: 140–142
Miyaura C, Onozaki K, Akiyama Y, Taniyama T, Hirano T, Kishimoto T and Suda T (1988) Recombinant human interleukin 6 (B-cell stimulatory factor 2) is a potent inducer of differentiation of mouse myeloid leukemia cells (M1). FEBS Lett. 234: 17–21
Peled A, Lee BC, Toledo J, Aracil M and Zipori D (1996) Interactions between leukemia cells and bone marrow stromal cells: stroma-supported growth vs. serum dependence and the roles of TGF-β and M-CSF. Exp. Hematol. 24: 728–737
Matas D, Sigal A, Stambolsky P, Milyavsky M, Weisz L, Schwartz D, Goldfinger N and Rotter V (2001) Integrity of the N-terminal transcription domain of p53 is required for mutant p53 interference with drug-induced apoptosis. EMBO J. 20: 4163–4172
Zhang W, Guo XY, Hu GY, Liu WB, Shay JW and Deisseroth AB (1994) A temperature-sensitive mutant of human p53. EMBO J. 13: 2535–2544
Yonish-Rouach E, Resnitzky K, Lotem J, Sachs L, Kimchi A and Oren M (1991) Wild type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin 6. Nature 352: 345–347
Darzynkiewicz Z, Li X and Gong J (1994) Assays of cell viability: Discrimination of cell dying by apoptosis. Methods Cell Biol. 41: 15–38
Lin J, Chen J, Elenbaas B and Levine AJ (1994) Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8: 1235–1246
Nozawa K, Maehara K and Isobe K (2001) Mechanism for the reduction of telomerase expression during muscle cell differentiation. J. Biol. Chem. 276: 22016–22023
Mattson MP and Klapper W (2001) Emerging roles for telomerase in neuronal development and apoptosis. J. Neurosci. Res. 63: 1–9
Wege H, Chui MS, Le HT, Tran JM and Zern MA (2003) SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 31: E3–3
Vousden KH (2000) p53: death star. Cell 103: 691–694
Brenner L, Teresita M-A, Vellucci VF, Zhou Z-L and Reiss M (1993) Wild-type p53 tumor suppressor gene restores differentiation of human squamous carcinoma cells but not the response to transforming growth factor β1. Cell Growth Differ. 4: 993–1004
Woodworth CD, Wang H, Simpson S, Alvarez-Salas LM and Notario V (1993) Overexpression of wild-type p53 alters growth and differentiation of normal human keratinocytes but not human papillomavirus-expressing cell lines. Cell Growth Differ. 4: 367–376
Spandau DF (1994) Distinct conformations of p53 are observed in different stages of keratinocyte differentiation. Oncogene 9: 1861–1868
Rehberger PA, Richter KH, Schwartz D, Goldfinger N, Oskato R, Almog N, Marks F and Rotter V (1997) Differential expression of the regularly spliced wild-type p53 and its COOH-terminal alternatively spliced form during epidermal differentiation. Cell Growth Differ. 8: 851–860
Almog N and Rotter V (1997) Involvement of p53 in cell differentiation and developmemt. Biochim. Biopyhs. Acta 1333: F1–F27
Caelles C, Helmberg A and Karin M (1994) p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370: 220–223
Wagner AJ, Kokontis JM and Hay N (1994) Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21(waf1/cip1). Gene Dev. 8: 2817–2830
Haupt Y, Rowan S, Shaulian E, Vousden KH and Oren M (1995) Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 9: 2170–2183
Haupt Y, Rowan S, Shaulian E, Kazaz A, Vousden K, Oren and M (1997) p53 mediated apoptosis in HeLa cells: transcription dependent and independent mechanisms. Leukemia 11 (Suppl 3): 337–339
Yan Y, Shay JW, Wright WE and Mumby MC (1997) Inhibition of protein phosphatase activity induces p53-dependent apoptosis in the absence of p53 transactivation. J Biol Chem 272: 15220–15226
Ding HF, Lin YL, McGill G, Juo P, Zhu H, Blenis J, Yuan J and Fisher DE (2000) Essential role for caspase-8 in transcription-independent apoptosis triggered by p53 (in process citation). J. Biol. Chem. 275: 38905–38911
Kokontis JM, Wagner AJ, O'Leary M, Liao S and Hay N (2001) A transcriptional activation function of p53 is dispensable for and inhibitory of its apoptotic function. Oncogene 20: 659–668
Amanullah A, Liebermann DA and Hoffman B (2000) p53-independent apoptosis associated with c-Myc-mediated block in myeloid cell differentiation. Oncogene 19: 2967–2977
Amanullah A, Liebermann DA and Hoffman B (2002) Deregulated c-Myc prematurely recruits both Type I and II CD95/Fas apoptotic pathways associated with terminal myeloid differentiation. Oncogene 21: 1600–1610
Bennet M, Macdonald K, Chan S-W, Luzio JP, Simari R and Weissberg P (1998) Cell surface trafficking of fax: a rapid mechanism of p53-mediated apoptosis. Science 282: 290–293
Chen X, Ko LJ, Jayaraman L and Prives C (1996) p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev 10: 2438–2451
Offer H, Erez N, Zurer I, Tang X, Milyavsky M, Goldfinger N and Rotter V (2002) The onset of p53-dependent DNA repair or apoptosis is determined by the level of accumulated damaged DNA. Carcinogenesis 23: 1025–1032
Ronen D, Schwartz D, Teitz Y, Goldfinger N and Rotter V (1996) Induction of HL-60 cells to undergo apoptosis is determined by high levels of wild-type p53 protein whereas differentiation of the cells is mediated by lower p53 levels. Cell Growth Differ 7: 21–30
Miller Jr WH and Waxman S (2002) Differentiation induction as a treatment for hematologic malignancies. Oncogene 21: 3496–3506
Peled A, Zipori D and Rotter V (1996) Cooperation between p53-dependent and p53-independent apoptotic pathways in myeloid cells. Cancer Res 56: 2148–2156
Li R, Sutphin PD, Schwartz D, Matas D, Almog N, Wolkowicz R, Goldfinger N, Pei H, Prokocimer M and Rotter V (1998) Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene 16: 3269–3277
Acknowledgements
This study was supported in part by grants from the Israel–USA Binational Science Foundation (BSF), the Israeli Science Foundation (ISF) and the Kadoori Foundation. VR is the incumbent of the Norman and Helen Asher Professorial Chair in Cancer Research at the Weizmann Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editted by D Altieri
Rights and permissions
About this article
Cite this article
Matas, D., Milyavsky, M., Shats, I. et al. p53 is a regulator of macrophage differentiation. Cell Death Differ 11, 458–467 (2004). https://doi.org/10.1038/sj.cdd.4401379
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401379
Keywords
This article is cited by
-
NGF-mediated transcriptional targets of p53 in PC12 neuronal differentiation
BMC Genomics (2007)
-
P53 and Beta-Catenin Activity during Estrogen treatment of Osteoblasts
Cancer Cell International (2005)
-
Concepts of human leukemic development
Oncogene (2004)