Abstract
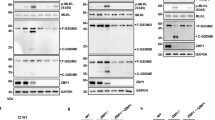
ZBP-89 induces apoptosis in human gastrointestinal cancer cells through a p53-independent mechanism. To understand the apoptotic pathway regulated by ZBP-89, we identified downstream signal transduction targets. Ectopic expression of ZBP-89 induced apoptosis through the mitochondrial pathway and was accompanied by activation of all three MAP kinase subfamilies: JNK1/2, ERK1/2 and p38 MAP kinase. ZBP-89-induced apoptosis was markedly enhanced by ERK inhibition with U0126. In contrast, inhibiting JNK with a JNK1-specific peptide inhibitor or dominant-negative JNK2 expression abrogated ZBP-89-mediated apoptosis. The p38 inhibitor SB202190 had no effect on ZBP-89-induced cell death. Protein dephosphorylation assays revealed that ZBP-89 activates JNK via repression of JNK dephosphorylation. Oligonucleotide microarray analyses revealed that ectopic expression of ZBP-89 downregulated expression of the dual-specificity phosphatase MKP6. Overexpression of MKP6 blocked ZBP-89-induced JNK phosphorylation and PARP cleavage. In addition, ectopic expression of ZBP-89 repressed Bcl-xL and Mcl-1 expression, but had no effect on Bcl-2. Silencing ZBP-89 with small interfering RNA enhanced both Bcl-xL and Mcl-1 expression. Taken together, ZBP-89-mediated apoptosis occurs via a p53-independent mechanism that requires JNK activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MAPK:
-
mitogen-activated protein kinase
- MEK:
-
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- ERK:
-
extracellular signal-regulated kinase
- JNK:
-
c-Jun N-terminal kinase
- PARP:
-
poly-(ADP ribose) polymerase
- Ad:
-
adenovirus
- dnJNK2:
-
dominant-negative c-Jun N-terminal kinase 2
- p-JNK:
-
phosphorylated c-Jun N-terminal kinase
- GFP:
-
green fluorescence protein
- siRNA:
-
small interfering RNA
- RNAi:
-
RNA interference
- JIP-1:
-
JNK-interacting protein-1
- DSP:
-
dual-specificity phosphatase
- MKP:
-
MAP kinase phosphatase
References
Hatano E, Bradham CA, Stark A, Iimuro Y, Lemasters JJ and Brenner DA (2000) The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J. Biol. Chem. 275: 11814–11823
Blagosklonny MV (2002) P53: an ubiquitous target of anticancer drugs. Int. J. Cancer 98: 161–166
Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, Warrington JA and King MC (2002) BRCA1 transcriptionally regulates genes involved in breast tumorigenesis. Proc. Natl. Acad. Sci. USA 99: 7560–7565
Schuler M and Green DR (2001) Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 29: 684–688
Ashkenazi A (2002) Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2: 420–430
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252
Ichijo H (1999) From receptors to stress-activated MAP kinases. Oncogene 18: 6087–6093
Erhardt P, Schremser EJ and Cooper GM (1999) B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19: 5308–5315
von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR and Troppmair J (2001) Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol. Cell. Biol. 21: 2324–2336
Graves JD, Draves KE, Craxton A, Saklatvala J, Krebs EG and Clark EA (1996) Involvement of stress-activated protein kinase and p38 mitogen-activated protein kinase in mIgM-induced apoptosis of human B lymphocytes. Proc. Natl. Acad. Sci. USA 93: 13814–13818
Kinoshita T, Yokota T, Arai K and Miyajima A (1995) Suppression of apoptotic death in hematopoietic cells by signalling through the IL-3/GM-CSF receptors. EMBO J. 14: 266–275
Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ and Davis RJ (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270: 7420–7426
Xia Z, Dickens M, Raingeaud J, Davis RJ and Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331
Tamura S, Hanada M, Ohnishi M, Katsura K, Sasaki M and Kobayashi T (2002) Regulation of stress-activated protein kinase signaling pathways by protein phosphatases. Eur. J. Biochem. 269: 1060–1066
Willoughby EA, Perkins GR, Collins MK and Whitmarsh AJ (2003) The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J. Biol. Chem. 278: 10731–10736
Merchant JL, Iyer GR, Taylor BR, Kitchen JR, Mortensen ER, Wang Z, Flintoft RJ, Michel J and Bassel-Duby R (1996) ZBP-89, a Krüppel-type zinc finger protein, inhibits EGF induction of the gastrin promoter. Mol. Cell. Biol. 16: 6644–6653
Bai L, Logsdon C and Merchant JL (2002) Regulation of epithelial cell growth by ZBP-89: potential relevance in pancreatic cancer. Int. J. Gastro. Can. 31: 79–88
Bai L and Merchant JL (2001) ZBP-89 promotes growth arrest through stabilization of p53. Mol. Cell. Biol. 21: 4670–4683
Remington MC, Tarle SA, Simon B and Merchant JL (1997) ZBP-89, a Kruppel-type zinc finger protein, inhibits cell proliferation. Biochem. Biophys. Res. Commun. 237: 230–234
Takeuchi A, Mishina Y, Miyaishi O, Kojima E, Hasegawa T and Isobe KI (2003) Heterozygosity with respect to Zfp148 causes complete loss of fetal germ cells during mouse embryogenesis. Nat. Genet. 33: 172–176
Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S, Weichselbaum R, Nalin C and Kufe D (1997) Role for Bcl-xL as an inhibitor of cytosolic cytochrome c accumulation in DNA damage-induced apoptosis. Proc. Natl. Acad. Sci. USA 94: 6939–6942
Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl- 2 regulation of apoptosis [see comments]. Science 275: 1132–1136
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, Umezawa A and Ichijo H (2002) Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J. Biol. Chem. 277: 43730–43734
Weber JD, Raben DM, Phillips PJ and Baldassare JJ (1997) Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J. 326: 61–68
Swank MW and Sweatt JD (2001) Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J. Neurosci. 21: 3383–3391
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M and Lord JM (2000) Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256: 34–41
Franklin RA and McCubrey JA (2000) Kinases: positive and negative regulators of apoptosis. Leukemia 14: 2019–2034
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y and Seger R (2001) Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 20: 147–155
Kibbe MR, Li J, Nie S, Choi BM, Kovesdi I, Lizonova A, Billiar TR and Tzeng E (2002) Potentiation of nitric oxide-induced apoptosis in p53−/− vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 282: C625–C634
Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C and Kufe D (2000) Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J. Biol. Chem. 275: 322–327
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288: 870–874
Meriin AB, YaglomJA, Gabai VL, Zon L, Ganiatsas S, Mosser DD and Sherman MY (1999) Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol. Cell. Biol. 19: 2547–2555
Willoughby EA, Perkins GR, Collins MK and Whitmarsh AJ (2003) The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J. Biol. Chem. 278: 10731–10736
Marti F, Krause A, Post NH, Lyddane C, Dupont B, Sadelain M and King PD (2001) Negative-feedback regulation of CD28 costimulation by a novel mitogen-activated protein kinase phosphatase, MKP6. J. Immunol. 166: 197–206
Chen YR, Wang X, Templeton D, Davis RJ and Tan TH (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271: 31929–31936
Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z and Kolesnick RN (1996) Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380: 75–79
Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF and Woodgett JR (1996) The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 6: 606–613
Caraglia M, Abbruzzese A, Leardi A, Pepe S, Budillon A, Baldassare G, Selleri C, Lorenzo SD, Fabbrocini A, Giuberti G, Vitale G, Lupoli G, Bianco AR and Tagliaferri P (1999) Interferon-alpha induces apoptosis in human KB cells through a stress-dependent mitogen activated protein kinase pathway that is antagonized by epidermal growth factor. Cell Death Differ. 6: 773–780
Lenczowski JM, Dominguez L, Eder AM, King LB, Zacharchuk CM and Ashwell JD (1997) Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol. Cell. Biol. 17: 170–181
Liu ZG, Hsu H, Goeddel DV and Karin M (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87: 565–576
Theodosiou A and Ashworth A (2002) Differential effects of stress stimuli on a JNK-inactivating phosphatase. Oncogene 21: 2387–2397
Weston CR and Davis RJ (2002) The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12: 14–21
Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD and Haber DA (1999) Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97: 575–586
Fornace Jr AJ, Jackman J, Hollander MC, Hoffman-Liebermann B and Liebermann DA (1992) Genotoxic-stress-response genes and growth-arrest genes. gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann. NY Acad. Sci. 663: 139–153
Hasegawa T, Xiao H and Isobe KI (1999) Cloning of a GADD34-like gene that interacts with the zinc-finger transcription factor which binds to the p21WAF promoter. Biochem. Biophys. Res. Commun. 256: 249–254
Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF and Ahn NG (1994) Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265: 966–970
Xia Z, Dickens M, Raingeaud J, Davis RJ and Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326–1331
Galve-Roperh I, Sanchez C, Cortes ML, del Pulgar TG, Izquierdo M and Guzman M (2000) Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 6: 313–319
Pumiglia KM and Decker SJ (1997) Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 94: 448–452
Ryan KM, Ernst MK, Rice NR and Vousden KH (2000) Role of NF-kappaB in p53-mediated programmed cell death. Nature 404: 892–897
Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW and DeFranco DB (2000) Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 275: 12200–12206
Yan Y, Haas JP, Kim M, Sgagias MK and Cowan KH (2002) BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. J. Biol. Chem. 24: 24
Giaccia AJ and Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12: 2973–2983
Lowe SW, Ruley HE, Jacks T and Housman DE (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967
Miyashita T and Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299
Oltvai ZN, Milliman CL and Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619
Deng X, Xiao L, Lang W, Gao F, Ruvolo P and May Jr MS (2001) Novel role for JNK as a stress-activated Bcl2 kinase. J. Biol. Chem. 276: 23681–23688
Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA and Chambers TC (2000) Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J. Biol. Chem. 275: 29980–29985
Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou JC and Arkinstall S (1997) Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J. Biol. Chem. 272: 25238–25242
Nishina H, Radvanyi L, Raju K, Sasaki T, Kozieradzki I and Penninger JM (1998) Impaired TCR-mediated apoptosis and Bcl-XL expression in T cells lacking the stress kinase activator SEK1/MKK4. J. Immunol. 161: 3416–3420
Yamamoto K, Ichijo H and Korsmeyer SJ (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol. Cell. Biol. 19: 8469–8478
Ando T, Umezawa A, Suzuki A, Okita H, Sano M, Hiraoka Y, Aiso S, Saruta T and Hata J (1998) EAT/mcl-1, a member of the bcl-2 related genes, confers resistance to apoptosis induced by cis-diammine dichloroplatinum (II) via a p53-independent pathway. Jpn. J. Cancer Res. 89: 1326–1333
Croxton R, Ma Y, Song L, Haura EB and Cress WD (2002) Direct repression of the Mcl-1 promoter by E2F1. Oncogene 21: 1359–1369
Lin MT, Juan CY, Chang KJ, Chen WJ and Kuo ML (2001) IL-6 inhibits apoptosis and retains oxidative DNA lesions in human gastric cancer AGS cells through up-regulation of anti-apoptotic gene mcl-1. Carcinogenesis 22: 1947–1953
Strasberg Rieber, Zangemeister-Wittke U and Rieber M (2001) p53-Independent induction of apoptosis in human melanoma cells by a bcl-2/bcl-xL bispecific antisense oligonucleotide. Clin. Cancer Res. 7: 1446–1451
Bai L and Merchant JL (2000) Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J. Biol. Chem. 275: 30725–30733
Harrington AW, Kim JY and Yoon SO (2002) Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J. Neurosci. 22: 156–166
Liu X, Kim CN, Yang J, Jemmerson R and Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K and Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498
Acknowledgements
The work was supported by Public Health Service NIH Grant DK 55732 to JLM. We thank the University of Michigan Cancer Center (5P30 CA46592) for use of the flow cytometry and vector cores. We also acknowledge use of the Michigan NIDDK Biotechnology Center (U24 DK58771). We thank Dr. Bert Vogelstein (Johns Hopkins University) for providing the HCT 116 p53 wild-type and null cell lines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by CJ Thiele
Rights and permissions
About this article
Cite this article
Bai, L., Yoon, S., King, P. et al. ZBP-89-induced apoptosis is p53-independent and requires JNK. Cell Death Differ 11, 663–673 (2004). https://doi.org/10.1038/sj.cdd.4401393
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401393
Keywords
This article is cited by
-
SGOL2 is a novel prognostic marker and fosters disease progression via a MAD2-mediated pathway in hepatocellular carcinoma
Biomarker Research (2022)
-
Understanding the regulation of β-catenin expression and activity in colorectal cancer carcinogenesis: beyond destruction complex
Clinical and Translational Oncology (2021)
-
Role of Bax/Bcl-2 family members in green tea polyphenol induced necroptosis of p53-deficient Hep3B cells
Tumor Biology (2014)
-
ZNF281/ZBP-99: a new player in epithelial–mesenchymal transition, stemness, and cancer
Journal of Molecular Medicine (2014)
-
SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition
The EMBO Journal (2013)