Abstract
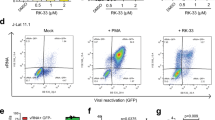
CD4+ T-cell death is a crucial feature of AIDS pathogenesis, but the mechanisms involved remain unclear. Here, we present in vitro findings that identify a novel process of HIV1 mediated killing of bystander CD4+ T cells, which does not require productive infection of these cells but depends on the presence of neighboring dying cells. X4-tropic HIV1 strains, which use CD4 and CXCR4 as receptors for cell entry, caused death of unstimulated noncycling primary CD4+ T cells only if the viruses were produced by dying, productively infected T cells, but not by living, chronically infected T cells or by living HIV1-transfected HeLa cells. Inducing cell death in HIV1-transfected HeLa cells was sufficient to obtain viruses that caused CD4+ T-cell death. The addition of supernatants from dying control cells, including primary T cells, allowed viruses produced by living HIV1-transfected cells to cause CD4+ T-cell death. CD4+ T-cell killing required HIV1 fusion and/or entry into these cells, but neither HIV1 envelope-mediated CD4 or CXCR4 signaling nor the presence of the HIV1 Nef protein in the viral particles. Supernatants from dying control cells contained CD95 ligand (CD95L), and antibody-mediated neutralization of CD95L prevented these supernatants from complementing HIV1 in inducing CD4+ T-cell death. Our in vitro findings suggest that the very extent of cell death induced in vivo during HIV1 infection by either virus cytopathic effects or immune activation may by itself provide an amplification loop in AIDS pathogenesis. More generally, they provide a paradigm for pathogen-mediated killing processes in which the extent of cell death occurring in the microenvironment might drive the capacity of the pathogen to induce further cell death.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CD95L:
-
CD95 (Fas) ligand
- ΔΨm:
-
mitochondrial transmembrane potential
- Env:
-
envelope protein
- GFP:
-
green fluorescent protein
- HIV:
-
human immunodeficiency virus
- mAb:
-
monoclonal antibody
- MuLV:
-
murine leukemia virus
- NL4-3/env:
-
vector encoding NL4-3 deleted in its env gene
- NL4-3/nef:
-
vector encoding NL4-3 deficient in its nef gene
- NL4-3/MuLV env:
-
NL4-3 viruses pseudotyped with the amphotropic MuLV env gene
- sCD4:
-
human soluble recombinant CD4
- SIV:
-
simian immunodeficiency virus
- srCD95L:
-
soluble recombinant FLAG-tagged CD95L
- TNF:
-
tumor necrosis factor
References
Shearer GM (1998) HIV-induced immunopathogenesis. Immunity 9: 587–593
Badley AD, Pilon AA, Landay A and Lynch DH (2000) Mechanisms of HIV-associated lymphocyte apoptosis. Blood 96: 2951–2964
Mc Cune JM (2001) The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410: 974–979
Grossman Z, Meier-Schellersheim M, Sousa A, Victorino R and Paul W (2002) CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8: 319–323
Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM and Markowitz M (1995) Rapid turnover of plasma virions and CD4+ lymphocytes in HIV-1 infection. Nature 373: 123–126
Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, Saag MS and Shaw GM (1995) Viral dynamics in HIV-1 infection. Nature 373: 117–122
Perelson AS, Neumann AU, Markowitz M, Leonard JM and Ho DD (1996) HIV-1 dynamics in vivo: virion clearance rate, infected-cell life span, and viral generation time. Science 271: 1582–1586
Ameisen JC and Capron A (1991) Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today 12: 102–105
Ameisen JC, Estaquier J, Idziorek T and De Bels F (1995) Programmed cell death and AIDS: significance, perspectives and unanswered questions. Cell Death Differ. 2: 9–22
Ameisen JC (1998) HIV: setting death in motion. Nature 395: 117–119
Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM and Kupper A (1995) Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1: 129–134
Muro-Cacho CA, Pantaleo G and Fauci AS (1995) Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with the stage of disease or viral burden. J. Immunol. 154: 5555–5566
Badley AD, Dockrell DH, Algeciras A, Ziesmer S, Landay A, Lederman MM, Connick E, Kessler H, Kuritzkes D, Lynch DH, Roche P, Yagita H and Paya CV (1998) In vivo analysis of Fas/FasL interactions in HIV-infected patients. J. Clin. Invest. 102: 79–87
Rosok B, Brinchmann JE, Stent G, Bjerknes R, Voltersvik P, Olofsson J and Asjo B (1998) Correlates of apoptosis of CD4+ and CD8+ T cells in tonsillar tissue in HIV type 1 infection. AIDS Res. Hum. Retrov. 14: 1635–1643
Wykrzykowska JJ, Rosenzweig M, Veazey RS, Simon MA, Halvorsen K, Desrosiers RC, Johnson RP and Lackner AA (1998) Early regeneration of thymic progenitors in rhesus macaques infected with SIV. J. Exp. Med. 11: 1767–1778
Jekle A, Keppler O, De Clercq E, Schols D, Weinstein M and Goldsmith MA (2003) In vivo evolution of HIV1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4+ T cells. J. Virol. 77: 5846–5854
Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guétard D, Rame V, Dauguet C and Montagnier L (1993) Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrov. 9: 553–563
Estaquier J, Idziorek T, De Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M and Ameisen JC (1994) Programmed cell death and AIDS: the significance of T-cell apoptosis in pathogenic and non pathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91: 9431–9435
Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P and Ameisen JC (1996) Fas-mediated apoptosis of CD4+ and CD8+ T cells from HIV-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87: 4959–4966
Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly RP and Finkel TH (1992) Crosslinking CD4 by HIV gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176: 1099–1106
Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM and Krammer PH (1995) Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375: 497–500
Accornero P, Radrizzani M, Delta D, Gerosa F, Kurrie R and Colombo MP (1997) Differential susceptibility to HIV gp120-sensitized apoptosis in CD4+ T-cell clones with different T-helper phenotypes: role of CD95/CD95L interactions. Blood 89: 558–569
Kameoka M, Kimura T, Zheng YH, Suzuki S, Fujinaga K, Luftig RB and Ikuta K (1997) Protease-defective gp120-containing HIV-1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy donor-derived peripheral blood T cells. J. Clin. Microbiol. 35: 41–47
Ohnimus H, Heinkelein M and Jassoy C (1997) Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF. J. Immunol. 159: 5246–5252
Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, De Clercq E and Este JA (1999) The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is dependent of the G protein-mediated signaling. AIDS 13: 909–917
Blanco J, Barretina J, Henson G, Bridger G, De Clercq E, Clotet B and Este JA (2000) The CXCR4 antagonist AMD3100 efficiently inhibits cell-surface-expressed HIV-1 envelope-induced apoptosis. Antimicrob. Agents Chemother. 44: 51–56
Desbarats J, Freed JH, Campbell PA and Newell MK (1996) Fas (CD95) expression and death-mediating function are induced by CD4 cross-linking on CD4+ T cells. Proc. Natl. Acad. Sci. USA 93: 11014–11018
Hashimoto F, Oyaizu N, Kalyanaraman VS and Pahwa S (1997) Modulation of Bcl-2 protein by CD4 cross-linking: a possible mechanism for lymphocyte apoptosis in HIV infection and for rescue of apoptosis by IL-2. Blood 90: 745–753
Algeciras A, Dockrell FH, Lynch DH and Paya CV (1998) CD4 regulates susceptibility to Fas ligand- and TNF-mediated apoptosis. J. Exp. Med. 187: 711–720
Berndt C, Mopps B, Angermuller S, Gierschik P and Krammer PH (1998) CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc. Natl. Acad. Sci. USA 95: 12556–12561
Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M and Goldsmith MA (2003) In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4+ T cells. J. Virol. 77: 5846–5854
Martin SJ, Matear P and Vyakarnam A (1994) HIV-1 infection of human CD4+ T cells in vitro. Differential induction of apoptosis in these cells. J. Immunol. 152: 330–342
Esser MT, Bess Jr. JW, Suryanarayana K, Chertova E, Marti D, Carrington M, Arthur LO and Lifson JD (2001) Partial activation and induction of apoptosis in CD4(+) and CD8(+) T lymphocytes by conformationally authentic noninfectious HIV1. J. Virol. 75: 1152–1164
Ducrey-Rundquist O, Guyader M and Trono D (2002) Modalities of IL7-induced HIV permissiveness in quiescent T lymphocytes. J. Virol. 76: 9103–9111
Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J and Stevenson M (2003) HIV1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424: 213–219
Dalgleish AG, Thomson BJ, Chanh TC, Malkovsky M and Kennedy RC (1987) Neutralisation of HIV isolates by anti-idiotypic antibodies which mimic the T4 (CD4) epitope: a potential AIDS vaccine. Lancet Ii: 1047–1050
Benkirane M, Corbeau P, Housset V and Devaux C (1993) An antibody that binds the immunoglobulin CDR3-like region of the CD4 molecule inhibits provirus transcription in HIV-infected T cells. EMBO J. 12: 4909–4921
Deen KC, McDougal JS, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon PJ, Axel R and Sweet RW (1988) A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature 331: 82–84
Bandres JC, Wang QF, O'Leary J, Baleaux F, Amara A, Hoxie JA, Zolla-Pazner S and Gorny MK (1998) HIV envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J. Virol. 72: 2500–2504
Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD and Katsikis PD (2001) Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 15: 871–882
Ostrowski MA, Justement SJ, Catanzaro A, Hallahan CA, Ehler LA, Mizell SB, Kumar PN, Mican JA, Chun TW and Fauci AS (1998) Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 161: 3195–3201
Murphy PM (2001) Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2: 116–122
Olah Z, Lehel C, Anderson WB, Eiden MV and Wilson CA (1994) The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 269: 25426–25431
van Zeijl M, Johann SV, Closs E, Cunningham J, Eddy R, Shows TB and O'Hara B (1994) A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc. Natl. Acad. Sci. USA 91: 1168–1172
Schaeffer E, Geleziunas R and Green WC (2001) HIV1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75: 2993–3000
Zauli G, Gibellini D, Secchiero P, Dutartre H, Olive D, Capitani S and Collette Y (1999) HIV1 Nef protein sensitizes CD4(+) T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood 93: 1000–1010
Geleziunas R, Xu W, Takeda K, Ichijo H and Greene WC (2001) HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410: 834–838
Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d'Aloja P, Schürmann A and Baur AS (2001) Nef-associated PAK and PI3-kinases stimulate Bad phosphorylation in an Akt-independent pathway. Implications for anti-apoptosis signaling in HIV-infected cells. Nat. Med. 7: 1217–1224
Ameisen JC (2001) Apoptosis subversion: HIV-Nef provides both armor and sword. Nat. Med. 7: 1181–1182
Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Naval J and Anel A (2001) Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J. Immunol. 187: 6736–6744
Martinez-Lorenzo MJ, Anel A, Gamen S, Monleon I, Lasierra P, Larrad L, Pineiro A, Alava MA and Naval J (1999) Activated human T cells release bioactive FasL and APO2 ligand in microvesicles. J. Immunol. 163: 1274–1281
Krammer P (2000) CD95's deadly mission in the immune system. Nature 407: 789–795
Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G and Fais S (2002) Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 195: 1303–1316
Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A and Tschopp J (1998) Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187: 1205–1213
Huang DCS, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J and Strasser A (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-XL. Proc. Natl. Acad. Sci. USA 96: 14871–14876
Li CJ, Friedman DJ, Wang C, Metelev V and Pardee AB (1995) Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268: 429–431
Bartz SR and Emerman M (1999) HIV-1 Tat induces apoptosis and increases sensitivity to apoptotic signals by upregulating FLICE/Caspase-8. J. Virol. 73: 1956–1963
Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M and Capitani S (1995) The HIV-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood. 86: 3823–3834
Chang HC, Samaniego F, Nair BC, Buonaguro L and Ensoli B (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic regions. AIDS 11: 1421–1431
Lenardo MJ, Angleman SB, Bounkeua V, Dimas J, Duvall MG, Graubard MB, Hornung F, Selkirk MC, Speirs CK, Trageser C, Orenstein JO and Bolton DL (2002) Cytopathic killing of peripheral blood CD4+ T lymphocytes by HIV-1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76: 5082–5093
Aupeix K, Hugel B, Martin T, Bischoff P, Lill H, Pasquali JL and Freyssinet JM (1997) The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J. Clin. Invest. 99: 1546–1554
Hosaka N, Oyaizu N, Kaplan MH, Yagila H and Pahwa S (1998) Membrane and soluble forms of Fas (CD95) and FasL in peripheral blood mononuclear cells and in plasma from HIV-infected persons. J. Infect. Dis. 178: 1030–1039
O'Brien SJ and Moore JP (2000) The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol. Rev. 177: 99–111
Gervaix A, West D, Leoni LM, Richman DD, Wong-Staal F and Corbeil J (1997) A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 94: 4653–4658
Schwartz O, Maréchal V, Danos O and Heard JM (1995) HIV1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69: 4053–4059
Landau N, Page KA and Littman DR (1991) Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J. Virol. 65: 162–169
Belloc F, Dumain P, Boisseau MR, Jalloustre C, Reiffers J, Bernard P and Lacombe F (1994) A flow cytometric method using Hoechst 33342 and propidium iodide for simultaneous cell cycle analysis and apoptosis determination in unfixed cells. Cytometry 17: 59–65
Acknowledgements
We thank A Fontana (Department of Internal Medicine, Zurich, Switzerland), A Gervaix (University of Geneva, Switzerland), D Klatzmann (CNRS ERS 107-CERVI, CHU Pitié Salpetrière, Paris, France), N Landau (Aaron Diamond Center, New York, NY, USA), P Schneider (Institute of Biochemistry, Epalinges, Switzerland), O Schwartz (Institut Pasteur, Paris, France), and K Strebel (NIH, Bethesda, MD, USA) for the generous gift of valuable reagents, and F Clavel (INSERM U552, CHU Bichat, Paris, France) for discussion. This work was supported by INSERM, Université Paris 7, Université Paris 7 Valorisation, AP-HP, and grants from ANRS, ECS, FRM, and EFG (to JCA), and doctoral fellowships from ANRS (to JDL), ECS (to FP), and DGA and FRM (to DA).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Lelièvre, J., Mammano, F., Arnoult, D. et al. A novel mechanism for HIV1-mediated bystander CD4+ T-cell death: neighboring dying cells drive the capacity of HIV1 to kill noncycling primary CD4+ T cells. Cell Death Differ 11, 1017–1027 (2004). https://doi.org/10.1038/sj.cdd.4401441
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401441
Keywords
This article is cited by
-
HIV integrase and the swan song of the CD4 T cells?
Retrovirology (2013)
-
IL-2 immunotherapy in chronically SIV-infected Rhesus Macaques
Virology Journal (2012)
-
CD95 ligand-dependant endothelial cell death initiates oral mucosa damage in a murine model of acute graft versus host disease
Laboratory Investigation (2007)