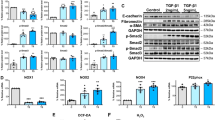
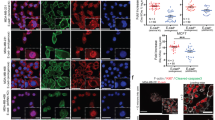
Abstract
Epithelial–mesenchymal transdifferentiation (EMT) is a critical morphogenic event that occurs during embryonic development and during the progression of various epithelial tumors. EMT can be induced by transforming growth factor (TGF)-β in mouse NMuMG mammary epithelial cells. Here, we demonstrate a central role of helix–loop–helix factors, E2A and inhibitor of differentiation (Id) proteins, in TGF-β-induced EMT. Epithelial cells ectopically expressing E2A adopt a fibroblastic phenotype and acquire migratory/invasive properties, concomitant with the suppression of E-cadherin expression. Id proteins interacted with E2A proteins and antagonized E2A-dependent suppression of the E-cadherin promoter. Levels of Id proteins were dramatically decreased by TGF-β. Moreover, NMuMG cells overexpressed Id2 showed partial resistance to TGF-β-induced EMT. Id proteins thus inhibit the action of E2A proteins on the expression of E-cadherin, but after TGF-β stimulation, E2A proteins are present in molar excess of the Id proteins, thus over-riding their inhibitory function and leading to EMT.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TGF-β:
-
transforming growth factor-β
- TβR-I:
-
TGF-β type I receptor
- BMPs:
-
bone morphogenetic proteins
- HLH:
-
helix–loop–helix
- Id:
-
inhibitor of differentiation
- EMT:
-
epithelial–mesenchymal transdifferentiation
- Tet:
-
tetracycline
- TRITC:
-
tetramethylrhodamine B isocyanate
- DMEM:
-
Dulbecco's modified Eagle's medium
- FBS:
-
fetal bovine serum
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
References
Shi Y and Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700
Miyazono K, Suzuki H and Imamura T (2003) Regulation of TGF-β signaling and its roles in progression of tumors. Cancer Sci. 94: 230–234
Derynck R, Akhurst RJ and Balmain A (2001) TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 29: 117–129
Wakefield LM and Roberts AB (2002) TGF-β signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12: 22–29
Miyazawa K, Shinozaki M, Hara T, Furuya T and Miyazono K (2002) Two major Smad pathways in TGF-β superfamily signalling. Genes Cells 7: 1191–1204
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2: 442–454
Savagner P (2001) Leaving the neighborhood: molecular mechanisms involved during epithelial–mesenchymal transition. BioEssays 23: 912–923
Bottinger EP and Bitzer M (2002) TGF-β signaling in renal disease. J. Am. Soc. Nephrol. 13: 2600–2610
Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA and Klewer SE (2002) Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 248: 170–181
Kaartinen V, Cui XM, Heisterkamp N, Groffen J and Shuler CF (1997) Transforming growth factor-β3 regulates transdifferentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev. Dyn. 209: 255–260
Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M, Hill CS, Beug H and Downward J (2000) Raf induces TGFβ production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14: 2610–2622
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H and Grunert S (2002) Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156: 299–313
Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H and Mikulits W (2002) Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-β1 and Ha-Ras: steps towards invasiveness. J. Cell Sci. 115: 1189–1202
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2: 76–83
Peinado H, Quintanilla M and Cano A (2003) Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial–mesenchymal transitions. J. Biol. Chem. 278: 21113–21123
Kee BL, Quong MW and Murre C (2000) E2A proteins: essential regulators at multiple stages of B-cell development. Immunol. Rev. 175: 138–149
Funato N, Ohtani K, Ohyama K, Kuroda T and Nakamura M (2001) Common regulation of growth arrest and differentiation of osteoblasts by helix–loop–helix factors. Mol. Cell. Biol. 21: 7416–7428
Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA and Cano A (2001) A new role for E12/E47 in the repression of E-cadherin expression and epithelial–mesenchymal transitions. J. Biol. Chem. 276: 27424–27431
Norton JD (2000) ID helix–loop–helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 113 (Part 22): 3897–3905
Miyazono K and Miyazawa K (2002) Id: a target of BMP signaling. Sci. STKE, PE40
Kang Y, Chen CR and Massague J (2003) A self-enabling TGFβ response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 11: 915–926
Piek E, Moustakas A, Kurisaki A, Heldin CH and ten Dijke P (1999) TGF-β type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J. Cell Sci. 112 (Part 24): 4557–4568
Akiyoshi S, Ishii M, Nemoto N, Kawabata M, Aburatani H and Miyazono K (2001) Targets of transcriptional regulation by transforming growth factor-β: expression profile analysis using oligonucleotide arrays. Jpn. J. Cancer Res. 92: 257–268
Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K and Miyazawa K (2003) SB-431542 and Gleevec inhibit transforming growth factor-β-induced proliferation of human osteosarcoma cells. Cancer Res. 63: 7791–7798
Lasorella A, Noseda M, Beyna M, Yokota Y and Iavarone A (2000) Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407: 592–598
Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, Inserra A and Iavarone A (2002) Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 62: 301–306
Siegel PM, Shu W and Massague J (2003) Mad upregulation and Id2 repression accompany TGF-β mediated epithelial cell growth suppression. J. Biol. Chem. 278: 35444–35450
Kee BL, Rivera RR and Murre C (2001) Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-β. Nat. Immunol. 2: 242–247
Lee MS, Lowe GN, Strong DD, Wergedal JE and Glackin CA (1999) TWIST, a basic helix–loop–helix transcription factor, can regulate the human osteogenic lineage. J. Cell. Biochem. 75: 566–577
Hebrok M, Fuchtbauer A and Fuchtbauer EM (1997) Repression of muscle-specific gene activation by the murine Twist protein. Exp. Cell Res. 232: 295–303
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J and Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2: 84–89
Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M and Cano A (2003) The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116: 499–511
Savagner P, Yamada KM and Thiery JP (1997) The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial–mesenchymal transition. J. Cell Biol. 137: 1403–1419
Hajra KM, Chen DY and Fearon ER (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 62: 1613–1618
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy F (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7: 1267–1278
Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL and Moses HL (2001) Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Cell. Biol. 12: 27–36
Kurisaki K, Kurisaki A, Valcourt U, Terentiev AA, Pardali K, Ten Dijke P, Heldin CH, Ericsson J and Moustakas A (2003) Nuclear factor YY1 inhibits transforming growth factor β- and bone morphogenetic protein-induced cell differentiation. Mol. Cell. Biol. 23: 4494–4510
Yokota Y, Mori S, Narumi O and Kitajima K (2001) In vivo function of a differentiation inhibitor, Id2. IUBMB Life 51: 207–214
Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J and Laping NJ (2002) Identification of novel inhibitors of the transforming growth factor β1 (TGF-beta1) type 1 receptor (ALK5). J. Med. Chem. 45: 999–1001
Mizuide M, Hara T, Furuya T, Takeda M, Kusanagi K, Inada Y, Mori M, Imamura T, Miyazawa K and Miyazono K (2003) Two short segments of Smad3 are important for specific interaction of Smad3 with c-Ski and SnoN. J. Biol. Chem. 278: 531–536
Faure S, Lee MA, Keller T, ten Dijke P and Whitman M (2000) Endogenous patterns of TGFβ superfamily signaling during early Xenopus development. Development 127: 2917–2931
Morgenstern JP and Land H (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18: 3587–3596
Debruyne PR, Vermeulen SJ, Berx G, Pocard M, Correia da Rocha AS, Li X, Cirnes L, Poupon MF, van Roy FM and Mareel MM (2003) Functional and molecular characterization of the epithelioid to round transition in human colorectal cancer LoVo cells. Oncogene 22: 7199–7208
Shaw LM, Rabinovitz I, Wang HH, Toker A and Mercurio AM (1997) Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 91: 949–960
Acknowledgements
We thank Dr. N Funato for valuable discussions, and Drs. C Murre, B and H Weintraub for providing crucial reagents. We also thank T Imamura, M Kato, A Hanyu and all the members of the Molecular Pathology Laboratory for critical comments. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. KM is also supported by a VRIA grant from Boehringer Ingelheim. EC is supported by a fellowship from the Carlos III Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Dr H Ichijo
Rights and permissions
About this article
Cite this article
Kondo, M., Cubillo, E., Tobiume, K. et al. A role for Id in the regulation of TGF-β-induced epithelial–mesenchymal transdifferentiation. Cell Death Differ 11, 1092–1101 (2004). https://doi.org/10.1038/sj.cdd.4401467
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401467
Keywords
This article is cited by
-
ID1 marks the tumorigenesis of pancreatic ductal adenocarcinoma in mouse and human
Scientific Reports (2022)
-
Foxf2 plays a dual role during transforming growth factor beta-induced epithelial to mesenchymal transition by promoting apoptosis yet enabling cell junction dissolution and migration
Breast Cancer Research (2018)
-
Id-1 promotes migration and invasion of non-small cell lung cancer cells through activating NF-κB signaling pathway
Journal of Biomedical Science (2017)
-
Downregulation of Lnc-Spry1 mediates TGF-β-induced epithelial–mesenchymal transition by transcriptional and posttranscriptional regulatory mechanisms
Cell Death & Differentiation (2017)
-
A 3D in vitro model to explore the inter-conversion between epithelial and mesenchymal states during EMT and its reversion
Scientific Reports (2016)