Abstract
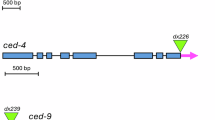
A genetically defined pathway orchestrates the removal of 131 of the 1090 somatic cells generated during the development of the hermaphrodite nematode Caenorhabditis elegans. Regulation of apoptosis is highly evolutionarily conserved and the nematode cell death pathway is a valuable model for studying mammalian apoptotic pathways, the dysregulation of which can contribute to numerous diseases. The nematode caspase CED-3 is ultimately responsible for the destruction of worm cells in response to apoptotic signals, but it must first be activated by CED-4. CED-9 inhibits programmed cell death and considerable data have demonstrated that CED-9 can directly bind and inhibit CED-4. However, it has been suggested that CED-9 may also directly inhibit CED-3. In this study, we used a yeast-based system and biochemical approaches to explore this second potential mechanism of action. While we confirmed the ability of CED-9 to inhibit CED-4, our data argue that CED-9 can not directly inhibit CED-3.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AFC:
-
7-amino-4-(trifluoromethyl)coumarin
- Apaf-1:
-
apoptotic protease activating factor-1
- Bcl-2:
-
B-cell lymphoma-2
- GFP:
-
green fluorescent protein
- GST:
-
glutathione-S-transferase
- PCR:
-
polymerase chain reaction
References
Sulston JE, Schierenberg E, White JG and Thomson JN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119
Yuan JY, Shaham S, Ledoux S, Ellis HM and Horvitz HR (1993) The C. elegans cell death gene ced 3 encodes a protein similar to mammalian interleukin-1-beta-converting enzyme. Cell 75: 641–652
Xue D, Shaham S and Horvitz HR (1996) The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 10: 1073–1083
Salvesen GS (2002) Caspases and apoptosis. Essays Biochem. 38: 9–19
Sugimoto A, Friesen PD and Rothman JH (1994) Baculovirus p35 prevents developmentally programmed cell death and rescues a ced 9 mutant in the nematode Caenorhabditis elegans. EMBO J. 13: 2023–2028
Xue D and Horvitz HR (1995) Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature 377: 248–251
Ellis HM and Horvitz HR (1986) Genetic control of programmed cell death in the nematode C. elegans. Cell 44: 817–829
Shaham S and Horvitz HR (1996) Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes Dev. 10: 578–591
Wu D, Wallen H, Inohara N and Nunez G (1997) Interaction and regulation of the Caenorhabditis elegans death protease CED-3 by CED-4 and CED-9. J. Biol. Chem. 272: 21449–21454
Chinnaiyan AM, Orourke K, Lane BR and Dixit VM (1997) Interaction of CED-4 with CED-3 and CED-9 – a molecular framework for cell death. Science 275: 1122–1126
Chinnaiyan AM, Chaudhary D, O'Rourke K, Koonin EV and Dixit VM (1997) Role of CED-4 in the activation of CED-3. Nature 388: 728–729
Irmler M, Hofmann K, Vaux D and Tschopp J (1997) Direct physical interaction between the Caenorhabditis elegans death proteins CED-3 and CED-4. FEBS. Lett. 406: 189–190
Yang X, Chang HY and Baltimore D (1998) Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science 281: 1355–1357
Hengartner MO, Ellis RE and Horvitz HR (1992) Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356: 494–499
Hengartner MO and Horvitz HR (1994) C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76: 665–676
Vaux DL, Weissman IL and Kim SK (1992) Prevention of programmed cell death in Caenorhabditis elegans by human Bcl-2. Science 258: 1955–1957
Wu DY, Wallen HD and Nunez G (1997) Interaction and regulation of subcellular localization of CED-4 by CED-9. Science 275: 1126–1129
Spector MS, Desnoyers S, Hoeppner DJ and Hengartner MO (1997) Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature 385: 653–656
Xue D and Horvitz HR (1997) Caenorhabditis elegans CED-9 protein is a bifunctional cell-death inhibitor. Nature 390: 305–308
Hawkins CJ, Wang SL and Hay BA (1999) A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc. Natl. Acad. Sci. USA 96: 2885–2890
Kang J, Schaber M, Srinivasula S, Alnemri E, Litwack G, Hall D and Bjornsti M (1999) Cascades of mammalian caspase activation in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274: 3189–3198
Hawkins CJ, Yoo SJ, Petersen EP, Wang SL, Vernooy SY and Hay BA (2000) The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J. Biol. Chem. 275: 27084–27093
Hawkins CJ, Wang SL and Hay BA (2000) Monitoring activity of caspases and their regulators in yeast Saccharomyces cerevisiae. Methods Enzymol. 322: 162–174
Hawkins CJ, Silke J, Verhagen AM, Foster R, Ekert PG and Ashley DM (2001) Analysis of candidate antagonists of IAP-mediated caspase inhibition using yeast reconstituted with the mammalian Apaf-1-activated apoptosis mechanism. Apoptosis 6: 331–338
Woo JS, Jung JS, Ha NC, Shin J, Kim KH, Lee W and Oh BH (2003) Unique structural features of a BCL-2 family protein CED-9 and biophysical characterization of CED-9/EGL-1 interactions. Cell Death Differ. 10: 1310–1319
Shibata M, Kanamori S, Ohsawa Y, Watanabe T, Yayoi Y, Miura M, Kominami E and Uchiyama Y (2001) Prevention of apoptosis of mammalian cells by the CED-3-cleaved form of CED-9. Arch. Histol. Cytol. 64: 17–28
Sikorski R and Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27
Wang SL, Hawkins CJ, Yoo SJ, Muller HA and Hay BA (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by REAPER, HID and GRIM, which disrupt DIAP1-caspase interactions. Cell 98: 453–463
Acknowledgements
We thank Trevor Lithgow and Lena Burri for their generous donation of the anti-GFP antibody and the p416-MET25-GFPS65 T plasmid. We thank David Vaux for providing helpful suggestions about the manuscript and for stimulating discussions. This study was supported by the Australian Research Council and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Nunez
Rights and permissions
About this article
Cite this article
Jabbour, A., Ho, Pk., Puryer, M. et al. The Caenorhabditis elegans CED-9 protein does not directly inhibit the caspase CED-3, in vitro nor in yeast. Cell Death Differ 11, 1309–1316 (2004). https://doi.org/10.1038/sj.cdd.4401501
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401501