Abstract
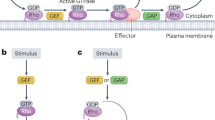
The current knowledge assigns a crucial role to the Rho GTPases family (Rho, Rac, Cdc42) in the complex transductive pathway leading to skeletal muscle cell differentiation. Their exact function in myogenesis, however, remains largely undefined. The protein toxin CNF1 was herein employed as a tool to activate Rho, Rac and Cdc42 in the myogenic cell line C2C12. We demonstrated that CNF1 impaired myogenesis by affecting the muscle regulatory factors MyoD and myogenin and the structural protein MHC expressions. This was principally driven by Rac/Cdc42 activation whereas Rho apparently controlled only the fusion process. More importantly, we proved that a controlled balance between Rho and Rac/Cdc42 activation/deactivation state was crucial for the correct execution of the differentiation program, thus providing a novel view for the role of Rho GTPases in muscle cell differentiation. Also, the use of Rho hijacking toxins can represent a new strategy to pharmacologically influence the differentiative process.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MRFs:
-
muscle-restricted regulatory factors
- SRF:
-
serum response factor
- CNF1:
-
cytotoxic necrotizing factor 1
- GM:
-
growth medium
- DM:
-
differentiation medium
- MHC:
-
myosin heavy chain
References
Molketin JD and Olson E (1996) Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6: 445–453
Perry LS and Rudnicki MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5: 750–767
Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD and Weintraub H (1989) MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 58: 823–831
Maione R and Amati P (1997) Interdependence between muscle differentiation and cell-cycle control. Biochim. Biophys. Acta 1332: M19–M30
Walsh K and Perlman H (1997) Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 7: 597–602
Wei Q and Paterson BM (2001) Regulation of MyoD function in the dividing myoblast. FEBS Lett. 490: 171–178
Etienne-Manneville S and Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635
Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N and Fernandez A (1998) RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9: 1891–1902
Wei L, Wei Z, Croissant JD, Johansen F-E, Prywes R, Balasubramanyam A and Schwartz RJ (1998) RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 273: 30284–30287
Gallo R, Serafini M, Castellani L, Falcone G and Alemà S (1999) Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell 10: 3137–3150
Meriane M, Roux P, Priming M, Fort P and Gauthier-Rouviere C (2000) Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 11: 2513–2528
Heller H, Gredinger E and Bengal E (2001) Rac1 inhibits myogenic dufferentiation by preventing the complete withdrawal of myoblasts from the cell cycle. J. Biol. Chem. 276: 37307–37316
Takano H, Komuro I, Oka T, Shiojima I, Hiuroi Y, Mizuno T and Yazaki Y (1998) The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18: 1580–1589
Fiorentini C, Gauthier M, Donelli G and Boquet P (1998) Bacterial toxins and the Rho GTP-binding protein: what microbes teach us about cell regulation. Cell Death Differ. 5: 720–728
Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C and Boquet P (1997) Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. J. Biol. Chem. 272: 19532–19537
Schmidt G, Sher P, Wilm M, Selzer J, Mann M and Aktories K (1997) Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387: 725–729
Lerm M, Schmidt G, Goehring UM, Schirmer J and Aktories K (1999) Identification of the region of Rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor 1 (CNF1). J. Biol. Chem. 274: 28999–29004
Fiorentini C, Falzano L, Fabbri A, Stringaro A, Logozzi M, Travaglione S, Contamin S, Arancia G, Malorni W and Fais S (2001) Activation of Rho GTPases by cytotoxic necrotizing factor 1 induces macropinocytosis and scavenging activity in epithelial cells. Mol. Biol. Cell 12: 2061–2073
Doye A, Mettouchi A, Bossis G, Clement R, Buisson-Touati C, Flatau G, Gagnoux L, Piechaczyk M, Boquet P and Lemichez E (2002) CNF1 exploits the ubiquitin–proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111: 553–564
Malorni W, Quaranta MG, Straface E, Falzano L, Fabbri A, Viora M and Fiorentini C (2003) The Rac-activating toxin cytotoxic necrotizing factor 1 oversees NK cell-mediated activity by regulating the actin/microtubule interplay. J. Immunol. 171: 4195–4202
Fiorentini C, Fabbri A, Flatau G, Donelli G, Matarrese P, Lemichez E, Falzano L and Boquet P (1997) Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 272: 19532–19537
Crescenzi M, Crouch DH and Tatò F (1994) Transformation by myc prevents fusion but not biochemical differentiation of C2C12 myoblasts: mechanisms of phenotypic correction in mixed culture with normal cells. J. Cell Biol. 125: 1137–1145
Russo S, Tomatis D, Collo G, Tarone G and Tatò F (1998) Myogenic conversion of NIH3T3 cells by exogenous MyoD family members: dissociation of terminal differentiation from myotube formation. J. Cell Sci. 111: 691–700
Hollenberg SM, Cheng PF and Weintraub H (1993) Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc. Natl. Acad. Sci. USA 90: 8028–8032
Essler M, Linder S, Scell B, Hufner K, Wiedemann A, Randhahn K, Staddon JM and Aepfelbacher M (2003) Cytotoxic necrotizing factor 1 of Escherichia coli stimulates Rho/Rho-kinase-dependent myosin light-chain phosphorylation without inactivating myosin light-chain phosphatase in endothelial cells. Infect. Immun. 71: 5188–5193
Moreau V, Tatin F, Varon C and Genot E (2003) Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol. Cell. Biol. 23: 6809–6822
Charrasse S, Meriane M, Comunale F, Blangy A and Gauthier-Rouviere C (2002) N- cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158: 935–965
Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M and Aktories K (1995) Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375: 500–503
Genth H, Gerhard R, Maeda A, Amano M, Kaibuchi K, Aktories K and Just I (2003) Entrapment of Rho ADP-ribosylated by Clostridium botulinum C3 exoenzyme in the Rho-guanine nucleotide dissociation inhibitor-1 complex. J. Biol. Chem. 278: 28523–28527
Ridley AJ and Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399
Ridley AJ, Paterson HF, Johnston CL, Diekmann D and Hall A (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70: 401–410
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA and Collard JG (1999) Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147: 1009–1022
Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1: 173–180
Noren NK, Niessen CM, Gumbiner BM and Burridge K (2001) Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276: 33305–33308
Arthur WT, Petch LA and Burridge K (2000) Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10: 719–722
Arthur WT and Burridge K (2001) RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12: 2711–2720
van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA and Collard JG (1999) Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1: 242–248
Weston C, Gordon C, Teressa G, Hod E, Ren XD and Prives J (2003) Cooperative regulation by Rac and Rho of agrin-induced acetylcholine receptor clustering in muscle cells. J. Biol. Chem. 278: 6450–6455
Meriane M, Charrasse S, Comunale F and Gauthier-Rouviere C (2002) Transforming growth factor beta activates Rac1 and Cdc42Hs GTPases and the JNK pathway in skeletal muscle cells. Biol. Cell 94: 535–543
Luo L, Liao YJ, Jan LY and Jan YN (1994) Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8: 1787–1802
Boyer L, Travaglione S, Falzano L, Gauthier NC, Popoff MR, Lemichez E, Fiorentini C and Fabbri A (2004) Rac GTPase instructs Nuclear Factor-kB activation by conveying the SCF complex and IkBα to the ruffling membranes. Mol. Biol. Cell 15: 1124–1133
Sordella R, Jiang W, Chen G-C, Curto M and Settleman J (2003) Modulation of Rho GTPase signaling regulated a switch between adipogenesis and myogenesis. Cell 113: 147–158
Kontaridis MI, Eminaga S, Fornaro M, Zito CI, Sordella R, Settleman J and Bennett AM (2004) SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signalling pathway. Mol. Cell. Biol. 24: 5340–5352
Joneson T, McDonough M, Bar-Sagi D and Van Aelst L (1996) Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science 274: 1374–1376
Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J and Hall A (1996) Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87: 519–529
Falzano L, Fiorentini C, Donelli G, Michel E, Kocks C, Cossart P, Cabanie L, Oswald E and Boquet P (1993) Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 9: 1247–1254
Bader D, Masaki T and Fischman DA (1982) Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell. Biol. 95: 763–770
Wright WE, Sasoon DA and Lin WK (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56: 607–617
Sander EE, van Delft S, ten Clooster JP, Reid T, van der Cammen RA, Michiels F and Collard JG (1998) Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidilynositol 3-kinase. J. Cell Biol. 143: 1385–1398
Ren XD, Kiosses WB and Schwartz MA (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18: 578–585
Russo S, Tatò F and Grossi M (1997) Transcriptional down-regulation of myogenin expression is associated with v-ras-induced block of differentiation in unestablished quail muscle cells. Oncogene 14: 63–73
Acknowledgements
We are grateful to W Malorni for critical reading of the manuscript and useful suggestions and to MG Quaranta for the densitometry analysis. The work has been partially supported by grants from ISS (project ‘Bacterial protein toxins as pharmacological agents that act on muscle cell differentiation’) to CF and from CNR/MIUR (project ‘Biomolecole per la salute umana’) and University of Rome ‘La Sapienza’ to MG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by R Knight
Rights and permissions
About this article
Cite this article
Travaglione, S., Messina, G., Fabbri, A. et al. Cytotoxic necrotizing factor 1 hinders skeletal muscle differentiation in vitro by perturbing the activation/deactivation balance of Rho GTPases. Cell Death Differ 12, 78–86 (2005). https://doi.org/10.1038/sj.cdd.4401522
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401522
Keywords
This article is cited by
-
RhoGTPases in stem cells
Chinese Science Bulletin (2007)