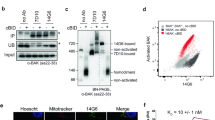
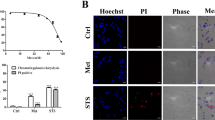
Abstract
Apoptosis represents an important cellular defence mechanism against viral pathogens by virtue of its ability to remove infected cells. Consequently, many viruses have developed numerous strategies to prevent or delay host cell apoptosis in order to achieve productive replication. Here we report that deletion of the F1L gene from the vaccinia genome results in increased apoptosis during infection. We demonstrate that F1L, which has no sequence homology to Bcl-2 family members, inhibits apoptosis at the level of mitochondria by binding to Bak. As a consequence, F1L prevents Bak activation, oligomerization and interaction with active Bax, all critical steps in the induction of apoptosis. We demonstrate that residues 64–84 of F1L interact directly with the Bcl-2 homology domain 3 (BH3) domain of Bak. This region of F1L has limited sequence similarity to known Bak-interacting BH3 domains. We also find that such additional BH3-like domains exist in the vaccinia genome. We conclude that F1L uses this specific, BH3-like domain to bind and inhibit Bak at the mitochondria.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BH3 domain:
-
Bcl-2 homology domain 3
- STS:
-
staurosporine
- TM:
-
transmembrane
- WR:
-
vaccinia virus Western Reserve
References
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev. 17: 2481–2495
Cory S and Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2: 647–656
Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DC (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17: 393–403
Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR and Newmeyer DD (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17: 525–535
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S and Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192
Roulston A, Marcellus RC and Branton PE (1999) Viruses and apoptosis. Annu. Rev. Microbiol. 53: 577–628
Boya P, Roques B and Kroemer G (2001) New EMBO members’ review: viral and bacterial proteins regulating apoptosis at the mitochondrial level. EMBO J. 20: 4325–4331
Cuconati A and White E (2002) Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 16: 2465–2478
Wasilenko ST, Meyers AF, Vander Helm K and Barry M (2001) Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75: 11437–11448
Wasilenko ST, Stewart TL, Meyers AF and Barry M (2003) Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 100: 14345–14350
Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E and Saikumar P (2003) Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278: 5367–5376
Nechushtan A, Smith CL, Lamensdorf I, Yoon SH and Youle RJ (2001) Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 153: 1265–1276
Stewart TL, Wasilenko ST and Barry M (2005) Vaccinia virus F1L protein is a tail-anchored protein that functions at the mitochondria to inhibit apoptosis. J. Virol. 79: 1084–1098
Petros AM, Olejniczak ET and Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644: 83–94
Wang G, Barrett JW, Nazarian SH, Everett H, Gao X, Bleackley C, Colwill K, Moran MF and McFadden G (2004) Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J. Virol. 78: 7097–7111
Liu X, Dai S, Zhu Y, Marrack P and Kappler JW (2003) The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19: 341–352
Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB and Fesik SW (1997) Structure of Bcl-xL–Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986
Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB and Fesik SW (2000) Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9: 2528–2534
Ploubidou A, Moreau V, Ashman K, Reckmann I, Gonzalez C and Way M (2000) Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 19: 3932–3944
Bartlett N, Symons JA, Tscharke DC and Smith GL (2002) The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 83 (Part 8): 1965–1976
Antonsson B, Montessuit S, Sanchez B and Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615–11623
Sundararajan R, Cuconati A, Nelson D and White E (2001) Tumor necrosis factor-alpha induces Bax–Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J. Biol. Chem. 276: 45120–45127
Griffiths GJ, Corfe BM, Savory P, Leech S, Esposti MD, Hickman JA and Dive C (2001) Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the pro-apoptotic protein Bak. Oncogene 20: 7668–7676
Fischer SF, Ludwig H, Holzapfel J, Kvansakul M, Chen L, Huang DC, Sutter G, Knese M and Hacker G (2006) Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 13: 109–118
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM and Huang DC (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19: 1294–1305
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB and Korsmeyer SJ (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14: 2060–2071
Cuconati A, Mukherjee C, Perez D and White E (2003) DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17: 2922–2932
Leu JI, Dumont P, Hafey M, Murphy ME and George DL (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak–Mcl1 complex. Nat. Cell Biol. 6: 443–450
Harada H, Quearry B, Ruiz-Vela A and Korsmeyer SJ (2004) Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc. Natl. Acad. Sci. USA 101: 15313–15317
Jeong SY, Gaume B, Lee YJ, Hsu YT, Ryu SW, Yoon SH and Youle RJ (2004) Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 23: 2146–2155
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR and Thompson CB (2000) The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6: 1389–1399
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730
Goldmacher VS (2005) Cell death suppression by cytomegaloviruses. Apoptosis 10: 251–265
Arnoult D, Bartle LM, Skaletskaya A, Poncet D, Zamzami N, Park PU, Sharpe J, Youle RJ and Goldmacher VS (2004) Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 101: 7988–7993
Poncet D, Larochette N, Pauleau AL, Boya P, Jalil AA, Cartron PF, Vallette F, Schnebelen C, Bartle LM, Skaletskaya A, Boutolleau D, Martinou JC, Goldmacher VS, Kroemer G and Zamzami N (2004) An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279: 22605–22614
Andoniou CE, Andrews DM, Manzur M, Ricciardi-Castagnoli P and Degli-Esposti MA (2004) A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J. Cell Biol. 166: 827–837
Rottger S, Frischknecht F, Reckmann I, Smith GL and Way M (1999) Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J. Virol. 73: 2863–2875
Hirt P, Hiller G and Wittek R (1986) Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J. Virol. 58: 757–764
Acknowledgements
We thank D Hancock (Cancer Research UK, London, UK) for Bcl-2, Bcl-XL, Bax and Bak cDNAs, and Eyad Eddaoudi, Gary Warnes and Sabrina Leverrier for help and advice with the FACS analyses. We also thank Mike Mitchell for help with the FuzzPro analysis, Jacomine Krijnse-Locker for the anti-F17R antibody and Nicola O’Reilly for peptide synthesis and preparation of the peptide array. AP was supported by a Marie Curie postdoctoral fellowship (HPMF-CT-2002–01590). We have no conflicting financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by A Villunger
Note added in proof
After submission of this paper, Wasilenko et al. reported that F1L binds Bak (J. Virol. 79, 14031, 2005).
Rights and permissions
About this article
Cite this article
Postigo, A., Cross, J., Downward, J. et al. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ 13, 1651–1662 (2006). https://doi.org/10.1038/sj.cdd.4401853
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401853
Keywords
This article is cited by
-
Viral pro-survival proteins block separate stages in Bax activation but changes in mitochondrial ultrastructure still occur
Cell Death & Differentiation (2008)
-
Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands
Cell Death & Differentiation (2008)
-
Iridovirus Bcl-2 protein inhibits apoptosis in the early stage of viral infection
Apoptosis (2008)
-
Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes
Apoptosis (2007)
-
Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis
Cell Death & Differentiation (2006)