Abstract
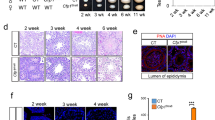
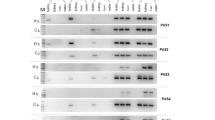
In a model of male sterility (MTp53) owing to enforced p53 expression in spermatocytes II and spermatids of transgenic mice, we focused on the role of caspases. Most of them are expressed in all differentiation stages, but only the transcriptional levels of caspase-2 and caspase-3 are modified in MTp53 germ cells. In normal testis, cleaved caspase-3 and caspase-9 are detected during the elongation of spermatids. Despite this constitutive presence of caspases during terminal differentiation, calpains are the main effectors of germ cell loss in MTp53 testes: calpain 1 RNA levels are increased, caspase-3-like activity is markedly decreased while calpain activity is higher and the calpain inhibitor E64d ((2S, 3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester) reduces TUNEL labeling in MTp53 testis, whereas pancaspase inhibitor zVADfmk (N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone) has no effect. Our work suggests that despite the presence, and potent involvement, of caspases in male haploid cell maturation, calpains are the executioners of the death of terminally differentiating germ cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MTI:
-
metallothionein
- SP:
-
side population
- CARD:
-
caspase recruitment domain
- zVADfmk:
-
N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
- E64d:
-
(2S, 3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester
References
Mueller T, Voigt W, Simon H, Fruehauf A, Bulankin A, Grothey A and Schmoll HJ (2003) Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Res. 63: 513–521.
Hasegawa M, Zhang Y, Niibe H, Terry N and Meistrich M (1998) Resistance of differentiating spermatogonia to radiation-induced apoptosis and loss in p53-deficient mice. Radiat. Res. 149: 263–270.
Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, Rutgers DH and de Rooij DG (1998) The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 5: 669–677.
Earnshaw WC, Martins LM and Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383–424.
Honarpour N, Du C, Richardson JA, Hammer RE, Wang X and Herz J (2000) Adult Apaf-1-deficient mice exhibit male infertility. Dev. Biol. 218: 248–258.
Shiraishi K, Naito K and Yoshida K (2000) Inhibition of calpain but not caspase protects the testis against injury after experimental testicular torsion of rat. Biol. Reprod. 63: 1538–1548.
Franco SJ and Huttenlocher A (2005) Regulating cell migration: calpains make the cut. J. Cell Sci. 118: 3829–3838.
Vandenabeele P, Orrenius S and Zhivotovsky B (2005) Serine proteases and calpains fulfill important supporting roles in the apoptotic tragedy of the cellular opera. Cell Death Differ. 12: 1219–1224.
Allemand I, Anglo A, Jeantet AY, Cerutti I and May E (1999) Testicular wild-type p53 expression in transgenic mice induces spermiogenesis alterations ranging from differentiation defects to apoptosis. Oncogene 18: 6521–6530.
De SK, Enders GC and Andrews GK (1991) High levels of metallothionein messenger RNAs in male germ cells of the adult mouse. Mol. Endocrinol. 5: 628–636.
Lassalle B, Bastos H, Louis JP, Riou L, Testart J, Dutrillaux B, Fouchet P and Allemand I (2004) ‘Side population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development 131: 479–487.
Bastos HLB, Chicheportiche A, Riou L, Testart J, Allemand I and Fouchet P (2005) Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry 65: 40–49.
Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U and Momoi T (1999) Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 264: 550–555.
Giampietri C, Petrungaro S, Coluccia P, D'Alessio A, Starace D, Riccioli A, Padula F, Srinivasula SM, Alnemri E, Palombi F, Filippini A, Ziparo E and De Cesaris P (2003) FLIP is expressed in mouse testis and protects germ cells from apoptosis. Cell Death Differ. 10: 175–184.
Wang L, Miura M, Bergeron L, Zhu H and Yuan J (1994) Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell 78: 739–750.
Kumar S, Kinoshita M, Loretta D and Makoto N (1997) Origin, expression and possible functions of the two alternatively spliced forms of the mouse Nedd2 mRNA. Cell Death Differ. 4: 378–387.
Johnson MD, Kinoshita Y, Xiang H, Ghatan S and Morrison RS (1999) Contribution of p53-dependent caspase activation to neuronal cell death declines with neuronal maturation. J. Neurosci. 19: 2996–3006.
Lankiewicz S, Marc Luetjens C, Truc Bui N, Krohn AJ, Poppe M, Cole GM, Saido TC and Prehn JH (2000) Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J. Biol. Chem. 275: 17064–17071.
Donovan M and Cotter TG (2002) Caspase-independent photoreceptor apoptosis in vivo and differential expression of apoptotic protease activating factor-1 and caspase-3 during retinal development. Cell Death Differ. 9: 1220–1231.
Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R and Faden AI (1997) Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17: 7415–7424.
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH and Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6: 797–801.
Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, Zhang MQ, Lazebnik Y, Bar-Sagi D and Lowe SW (2002) Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 4: 859–864.
Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, Chiorino G, Tagami H, Woo M and Dotto GP (2004) High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev. Cell 6: 551–562.
Solier S, Lansiaux A, Logette E, Wu J, Soret J, Tazi J, Bailly C, Desoche L, Solary E and Corcos L (2004) Topoisomerase I and II inhibitors control caspase-2 pre-messenger RNA splicing in human cells. Mol. Cancer Res. 2: 53–61.
Martinet W, Knaapen MW, De Meyer GR, Herman AG and Kockx MM (2003) Overexpression of the anti-apoptotic caspase-2 short isoform in macrophage-derived foam cells of human atherosclerotic plaques. Am. J. Pathol. 162: 731–736.
Houde C, Banks KG, Coulombe N, Rasper D, Grimm E, Roy S, Simpson EM and Nicholson DW (2004) Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of caspase-3-null mice. J. Neurosci. 24: 9977–9984.
Chua BT, Guo K and Li P (2000) Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J. Biol. Chem. 275: 5131–5135.
Chung SS, Wang X and Wolgemuth DJ (2005) Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation 73: 188–198.
Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H and Blomgren K (2005) The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia–ischemia. Cell Death Differ. 12: 162–176.
Wright KM, Linhoff MW, Potts PR and Deshmukh M (2004) Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J. Cell Biol. 167: 303–313.
Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC and Millan JL (2002) Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol. Cell Biol. 22: 5554–5562.
Cregan SP, Dawson VL and Slack RS (2004) Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23: 2785–2796.
Takano J, Tomioka M, Tsubuki S, Higuchi M, Iwata N, Itohara S, Maki M and Saido TC (2005) Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. J. Biol. Chem. 280: 16175–16184.
Tinel A and Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304: 843–846.
Droin N, Rebe C, Bichat F, Hammann A, Bertrand R and Solary E (2001) Modulation of apoptosis by procaspase-2 short isoform: selective inhibition of chromatin condensation, apoptotic body formation and phosphatidylserine externalization. Oncogene 20: 260–269.
Lamkanfi M, D'Hondt K, Vande Walle L, van Gurp M, Denecker G, Demeulemeester J, Kalai M, Declercq W, Saelens X and Vandenabeele P (2005) A novel caspase-2 complex containing TRAF2 and RIP1. J. Biol. Chem. 280: 6923–6932.
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL and Yuan J (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 12: 1304–1314.
Arama E, Agapite J and Steller H (2003) Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell 4: 687–697.
Huh JR, Vernooy SY, Yu H, Yan N, Shi Y, Guo M and Hay BA (2004) Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol. 2: E15.
Blanco-Rodriguez J and Martinez-Garcia C (1999) Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol. Reprod. 61: 1541–1547.
Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR and Steller H (2005) The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 8: 353–364.
Toppari J and Parvinen M (1985) In vitro differentiation of rat seminiferous tubular segments from defined stages of the epithelial cycle. Morphologic and immunolocalization analysis. J. Androl. 6: 334–343.
Launay S, Hermine O, Fontenay M, Kroemer G, Solary E and Garrido C (2005) Vital functions for lethal caspases. Oncogene 24: 5137–5148.
Bizat N, Hermel JM, Humbert S, Jacquard C, Creminon C, Escartin C, Saudou F, Krajewski S, Hantraye P and Brouillet E (2003) In vivo calpain/caspase cross-talk during 3-nitropropionic acid-induced striatal degeneration: implication of a calpain-mediated cleavage of active caspase-3. J. Biol. Chem. 278: 43245–43253.
Jiang ZH, Zhang WJ, Rao Y and Wu JY (1998) Regulation of Ich-1 pre-mRNA alternative splicing and apoptosis by mammalian splicing factors. Proc. Natl. Acad. Sci. USA 95: 9155–9160.
Acknowledgements
This work was supported by the Association pour la Recherche contre le Cancer (Grant No. 5796) and by Electricité De France. We thank Patrick Flament for his precious help in maintaining our transgenic strains. We specially thank S Chevillard, V Joulin, B Lassalle and L Riou for their support and helpful discussions on this work. We thank D Marsh for his useful critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by B Zhivotovsky
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Coureuil, M., Fouchet, P., Prat, M. et al. Caspase-independent death of meiotic and postmeiotic cells overexpressing p53: calpain involvement. Cell Death Differ 13, 1927–1937 (2006). https://doi.org/10.1038/sj.cdd.4401887
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cdd.4401887
Keywords
This article is cited by
-
Chronic low-dose exposure to a mixture of environmental endocrine disruptors induces microRNAs/isomiRs deregulation in mouse concomitant with intratesticular estradiol reduction
Scientific Reports (2017)
-
Molecular portrait of cisplatin induced response in human testis cancer cell lines based on gene expression profiles
Molecular Cancer (2007)
-
Up-regulation of CD95 (Apo-1/Fas) is associated with spermatocyte apoptosis during the first round of spermatogenesis in the rat
Apoptosis (2007)