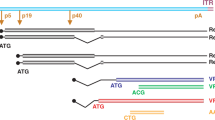
Abstract
The holy grail of gene therapy is the cure of genetic diseases. To achieve this goal, a vector system is desirable that offers a high level of safety combined with clinical efficacy and versatility in terms of potential applications. Gene therapy vectors based on recombinant adeno-associated viruses (AAVs) meet all of these criteria: They are nonpathogenic, devoid of viral coding sequences, and mediate long-term gene expression in the absence of an immune or inflammatory response. Moreover, with the recent discovery of novel AAV serotypes, there is now one preferred serotype for nearly every organ or tissue to target. Thus, AAV gene therapy vectors are increasingly becoming the vectors of choice for the treatment of inherited disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Schlehofer JR, Ehrbar M, zur Hausen H : Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology 1986; 152: 110–117.
Berns KI : Parvovirus replication. Microbiol Rev 1990; 54: 316–329.
Samulski RJ et al: Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell 1983; 33: 135–143.
Samulski RJ et al: Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA 1982; 79: 2077–2081.
Xie Q et al: The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 10405–10410.
Rutledge EA, Halbert CL, Russell DW : Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 1998; 72: 309–319.
Gao GP et al: Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Chiorini JA et al: Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol 1997; 71: 6823–6833.
Chiorini JA et al: Cloning and characterization of adeno-associated virus type 5. J Virol 1999; 73: 1309–1319.
Grimm D, Kay MA : From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 2003; 3: 281–304.
Leone P et al: Global CNS gene transfer for a childhood neurogenetic enzyme deficiency: Canavan disease. Curr Opin Mol Ther 1999; 1: 487–492.
Janson C et al: Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002; 13: 1391–1412.
Leone P et al: Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol 2000; 48: 27–38.
Gao G et al: Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA 2003; 100: 6081–6086.
Hildinger M et al: Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75: 6199–6203.
Grimm D : Production methods for gene transfer vectors based on adeno-associated virus serotypes. Methods 2002; 28: 146–157.
Kotin RM et al: Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA 1990; 87: 2211–2215.
Samulski RJ et al: Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J 1991; 10: 3941–3950.
Samulski RJ : Adeno-associated virus: integration at a specific chromosomal locus. Curr Opin Genet Dev 1993; 3: 74–80.
Nakai H et al: AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet 2003; 34: 297–302.
Russell DW : AAV loves an active genome. Nat Genet 2003; 34: 241–242.
Wright JF et al: Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr Opin Drug Discov Devel 2003; 6: 174–178.
Fisher KJ et al: Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 1997; 3: 306–312.
Yan Z et al: Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci USA 2000; 97: 6716–6721.
Reich SJ et al: Efficient trans-splicing in the retina expands the utility of adeno-associated virus as a vector for gene therapy. Hum Gene Ther 2003; 14: 37–44.
Halbert CL, Allen JM, Miller AD : Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol 2002; 20: 697–701.
Yakinoglu AO et al: DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res 1988; 48: 3123–3129.
McCarty DM, Monahan PE, Samulski RJ : Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Therapy 2001; 8: 1248–1254.
Donsante A et al: Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Therapy 2001; 8: 1343–1346.
Nickoloff JA : Transcription enhances intrachromosomal homologous recombination in mammalian cells. Mol Cell Biol 1992; 12: 5311–5318.
Prado F, Piruat JI, Aguilera A : Recombination between DNA repeats in yeast hpr1delta cells is linked to transcription elongation. EMBO J 1997; 16: 2826–2835.
High KA : Adeno-associated virus-mediated gene transfer for hemophilia B. Int J Hematol 2002; 76: 310–318.
Wagner JA et al: A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 2002; 13: 1349–1359.
Herzog RW et al: Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001; 4: 192–200.
During MJ et al: Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther 2001; 12: 1589–1591.
Fabb SA, Dickson JG : Technology evaluation: AAV factor IX gene therapy, Avigen Inc. Curr Opin Mol Ther 2000; 2: 601–606.
Wagner JA et al.: Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999; 109: 266–274.
Barranger JM, Novelli EA : Gene therapy for lysosomal storage disorders. Expert Opin Biol Ther 2001; 1: 857–867.
High KA, Theodore E : Woodward Award. AAV-mediated gene transfer for hemophilia. Trans Am Clin Climatol Assoc 2003; 114: 337–351, (discussion 351-2).
Auricchio A et al: A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther 2001; 4: 372–374.
Auricchio A et al: Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12: 71–76.
Loeb JE et al: Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999; 10: 2295–2305.
Kunzelmann K, Nitschke R : Defects in processing and trafficking of cystic fibrosis transmembrane conductance regulator. Exp Nephrol 2000; 8: 332–342.
Flotte TR et al: Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 1993; 268: 3781–3790.
Haberman RP, McCown TJ, Samulski RJ : Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J Virol 2000; 74: 8732–8739.
Flotte TR et al: Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther 2003; 14: 1079–1088.
Aitken ML et al: A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 2001; 12: 1907–1916.
Bell B, Canty D, Audet M : Hemophilia: an updated review. Pediatr Rev 1995; 16: 290–298.
Snyder RO et al: Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997; 16: 270–276.
Auricchio A et al: Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J Clin Invest 2002; 110: 499–504.
Manno CS et al: AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003; 101: 2963–2972.
Gordon N : Canavan disease: a review of recent developments. Eur J Paediatr Neurol 2001; 5: 65–69.
Baslow MH : Canavan's spongiform leukodystrophy: a clinical anatomy of a genetic metabolic CNS disease. J Mol Neurosci 2000; 15: 61–69.
Matalon RM, Michals-Matalon K : Spongy degeneration of the brain, Canavan disease: biochemical and molecular findings. Front Biosci 2000; 5: D307–D311.
Matalon R et al: Adeno-associated virus-mediated aspartoacylase gene transfer to the brain of knockout mouse for Canavan disease. Mol Ther 2003; 7: 580–587.
Alisky JM et al: Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport 2000; 11: 2669–2673.
Auricchio A : Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res 2003; 43: 913–918.
Stopa M : Gene therapy prospects in ophthalmology. Klin Oczna 2002; 104: 289–292.
Martin KR, Klein RL, Quigley HA : Gene delivery to the eye using adeno-associated viral vectors. Methods 2002; 28: 267–275.
Fazzi E et al: Leber's congenital amaurosis: an update. Eur J Paediatr Neurol 2003; 7: 13–22.
Dejneka NS, Rex TS, Bennett J : Gene therapy and animal models for retinal disease. Dev Ophthalmol 2003; 37: 188–198.
Cremers FP, van den Hurk JA, den Hollander AI : Molecular genetics of Leber congenital amaurosis. Hum Mol Genet 2002; 11: 1169–1176.
Perrault I et al: Leber congenital amaurosis. Mol Genet Metab 1999; 68: 200–208.
Acland GM et al: Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001; 28: 92–95.
Weber M et al: Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther 2003; 7: 774–781.
Acknowledgements
AA is recipient of fundings from the Italian Ministry of the University and of the Scientific and Technological Research (FIRB Gene Therapy), from the Italian Telethon Foundation, from The Ruth and Milton Steinbach Fund, from the Regione Campania and from the NIH (NEI, 1R01EY015136-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hildinger, M., Auricchio, A. Advances in AAV-mediated gene transfer for the treatment of inherited disorders. Eur J Hum Genet 12, 263–271 (2004). https://doi.org/10.1038/sj.ejhg.5201153
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201153
Keywords
This article is cited by
-
Illuminating the Gateway of Gene Silencing: Perspective of RNA Interference Technology in Clinical Therapeutics
Molecular Biotechnology (2012)
-
Efficacy of a Combined Intracerebral and Systemic Gene Delivery Approach for the Treatment of a Severe Lysosomal Storage Disorder
Molecular Therapy (2011)
-
Differential Transduction Following Basal Ganglia Administration of Distinct Pseudotyped AAV Capsid Serotypes in Nonhuman Primates
Molecular Therapy (2010)
-
Biochemical, Pathological, and Skeletal Improvement of Mucopolysaccharidosis VI After Gene Transfer to Liver but Not to Muscle
Molecular Therapy (2008)
-
Intron Splicing–mediated Expression of AAV Rep and Cap Genes and Production of AAV Vectors in Insect Cells
Molecular Therapy (2008)