Abstract
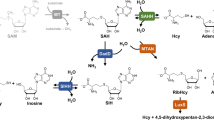
S-adenosylhomocysteine hydrolase (AdoHcyase) catalyzes the hydrolysis of AdoHcy to adenosine and homocysteine. Increased levels of AdoHcy may play a role in the development of cardiovascular diseases and numerous other conditions associated with hyperhomocysteinemia. Several polymorphic isoforms named SAHH-1 to 4 may be resolved by horizontal starch gel electrophoresis from red blood cells. We have identified the genetic background of isoforms SAHH-2 and SAHH-3. SAHH-2 represents the previously described polymorphism in exon 2 of the AdoHcyase gene (112 C>T; p.R38W). Isoform SAHH-3 is based on a new polymorphism in exon 3 (377 G>A), leading to the conversion of glycine to arginine at amino-acid position 123. To shed light on the effects of these polymorphisms on the molecular and catalytic properties of AdoHcyase, we made recombinant wild-type and polymorphic R38W and G123R enzymes for a comparative analysis. The amino-acid exchanges did not bring about major changes to the catalytic rates of the recombinant proteins. However, circular dichroism analysis showed that both polymorphisms effect the thermal stability of the recombinant protein in vitro, reducing the unfolding temperature by approximately 2.6°C (R38W) and 1.5°C (G123R) compared to wild-type protein. In view of the altered thermal stability, and slightly decreased enzymatic activity of polymorphic proteins (≤6%), one may consider the analyzed AdoHcyase isoforms as risk markers for diseases caused by irregular AdoHcyase metabolism.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gellekink H, den Heijer M, Kluijtmans LAJ et al: Effect of genetic variation in the human S-adenosylhomocysteine hydrolase gene on total homocysteine concentrations and risk of recurrent venous thrombosis. Eur J Hum Genet 2004; 12: 942–948.
Castro R, Rivera I, Blom HJ et al: Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J Inherit Metab Dis 2006; 29: 3–20.
Prigge ST, Chiang PK : S-adenosylhomocysteine hydrolase. in Carmel R, Jacobsen DW (eds): Homocysteine in Health and Disease. USA: Cambridge University Press, 2001, pp 79–90.
Loscalzo J : Homocysteine trials – clear outcomes for complex reasons. N Engl J Med 2006; 354: 1629–1632.
Dela Haba G, Cantoni GL : The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem 1959; 234: 603–608.
Coulter-Karis DE, Hershfield MS : Sequence of full length cDNA for human S-adenosylhomocysteine hydrolase. Ann Hum Genet 1989; 53: 169–175.
Palmer JL, Abeles RH : Mechanism for enzymatic thioether formation: mechanism of action of S-adenosylhomocysteinase. J Biol Chem 1976; 251: 5817–5819.
Turner MA, Yuan CS, Borchardt RT et al: Structure determination of selenomethionyl S-adenosylhomocysteine hydrolase using data at a single wavelength. Nat Struct Biol 1998; 5: 369–376.
Yamada T, Takata Y, Komoto J et al: Catalytic mechanism of S-adenosylhomocysteine hydrolase: Roles of His 54, Asp130, Glu155, Lys185, and Aspl89. Int J Biochem Cell Biol 2002; 37: 2417–2435.
Barić I, Fumić K, Glenn B et al: S-adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc Natl Acad Sci USA 2004; 101: 4234–4239.
Barić I, Ćuk M, Fumić K et al: S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy. J Inh Metab Dis 2005; 28: 885–902.
Buist NRM, Glenn B, Vugrek O et al: S-adenosylhomocysteine hydrolase deficiency in a 26-year-old man. J Inh Metab Dis 2006; 29: 538–545.
Kloor D, Fumić K, Attig S et al: Studies of S-adenosylhomocysteine-hydrolase polymorphism in a Croatian population. J Hum Genet 2006; 51: 21–24.
Belužić R, Ćuk M, Pavkov T et al: A single mutation at tyrosine 143 of human S-adenosylhomocysteine hydrolase renders the enzyme thermosensitive and effects the oxidation state of bound co-factor NAD. Biochem J 2006; 400: 245–253.
Takata Y, Yamada T, Huang Y et al: Catalytic mechanism of S-adenosylhomocysteine hydrolase: site-directed mutagenesis of Asp-130, Lys-185, Asp-189, and Asn-190. J Biol Chem 2002; 277: 22670–22676.
Hohman RJ, Guitton MC, Veron M : Inactivation of S-adenosyl-L-homocysteine hydrolase by cAMP results from dissociation of enzyme-bound NAD+. Proc Natl Acad Sci USA 1985; 82: 4578–4581.
Guex N, Peitsch MC : SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 1997; 18: 2714–2723.
Matuszewska B, Borchardt RT : The role of nicotinamide adenine dinucleotide in the inhibition of bovine liver S-adenosylhomocysteine hydrolase by Neplanocin A. J Biol Chem 1987; 262: 265–268.
Gomi T, Date T, Ogawa H et al: Expression of rat liver S-adenosylhomocysteinase cDNA in Escherichia coli and mutagenesis at the putative NAD binding site. J Biol Chem 1989; 264: 16138–16142.
Ault-Riche DB, Yuan CS, Borchardt RT : A single mutation at lysine 426 of human placental S-adenosylhomocysteine hydrolase inactivates the enzyme. J Biol Chem 1994; 269: 31472–31478.
Abeles RH, Fish S, Lipinskas B : S-adenosylhomocysteinase: mechanism of inactivation by 2′-deoxyadenosine and interaction with other nucleosides. Biochemistry 1982; 21: 5557–5562.
Corbo RM, Ingianna R, Scacchi R, Bozzi A : Kinetic properties of the common electrophoretic variants of human S-adenosylhomocysteine hydrolase (AHCY): the effect of four nucleoside analogue inhibitors. Ann Hum Genet 1992; 56: 35–43.
Acknowledgements
This work was supported by Grants 0098086 (OV) and 0108016 (KF, MC, IB) of the Ministry of Science, Education and Sports from the Republic of Croatia and by the Austrian Science Fund (FWF) project P17885 (TP). We thank Drs Dorotea Dorčić and Danijela Petković for help in collecting the samples and information of studied individuals. We are extremely grateful to Vesna Musani for supervision of the sequencing core facility at the Ruđer Bošković Institute. We greatly appreciate the commitment of participants and their informed consent regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fumić, K., Belužić, R., Ćuk, M. et al. Functional analysis of human S-adenosylhomocysteine hydrolase isoforms SAHH-2 and SAHH-3. Eur J Hum Genet 15, 347–351 (2007). https://doi.org/10.1038/sj.ejhg.5201757
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201757
Keywords
This article is cited by
-
Identification of enzymes and activity from two-dimensional gel electrophoresis
Nature Protocols (2007)