Abstract
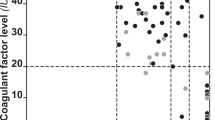
Factor VIII gene, F8, mutations cause haemophilia A (HA), an X-linked recessive disorder. Expression in heterozygous females has been ascribed to skewed X-chromosome inactivation (XCI). To investigate the cause of HA in three heterozygous females within an Atlantic Canadian kindred, the proband (severely affected girl, FVIII activity: 2%) and 17 relatives across three generations were studied. F8 genotype, FVIII activity, XCI ratio (XCIR) (paternal active X: maternal active X), karyotype, submegabase resolution tiling set array competitive genome hybridization (competitive genomic hybridization (SMRT)), and microsatellite analyses were utilized. A positive linear relationship between FVIII activity and percentage-activated normal X-chromosome was found in HA heterozygous females (R2=0.87). All affected, but no unaffected females, had an XCIR skewed toward activation of the mutant X-chromosome (proband 92:8, SD 2). Unexpectedly, high numbers of females have dramatically skewed XCIRs (>80:20 or <20:80) (P<0.05). The distribution of XCIR frequencies within this family was significantly different than predicted by normal population data or models of random XCI (P<0.025), with more females having higher degrees of skewing. Known causes of skewing, such as chromosomal abnormalities, selection against deleterious alleles, and X-inactive-specific transcript mutations, are not consistent with our results. This study shows that FVIII activity in HA heterozygous females can be directly related to XCI skewing, and that low FVIII activity in females in this family is due to unfavourable XCI skewing. Further, the findings suggest that these XCI ratios are genetically influenced, consistent with a novel heritable human X controlling element (XCE) functioning similarly to the mouse Xce.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Acquila M, Caprino D, Bicocchi P, Mori PG, Tagliaferri AR : A skewed lyonization phenomenon as cause of hemophilia A in a female patient. Blood 1995; 85: 599–600.
Bicocchi MP, Migeon BR, Pasino M et al: Familial nonrandom inactivation linked to the X inactivation centre in heterozygotes manifesting haemophilia A. Eur J Hum Genet 2005; 13: 635–640.
Lyon MF, Rastan S : Parental source of chromosome imprinting and its relevance for X chromosome inactivation. Differentiation 1984; 26: 63–67.
Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N : Requirement for Xist in X chromosome inactivation. Nature 1996; 379: 131–137.
Amos-Landgraf JM, Cottle A, Plenge RM et al: X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet 2006; 79: 493–499.
Naumova AK, Olien L, Bird LM et al: Genetic mapping of X-linked loci involved in skewing of X chromosome inactivation in the human. Eur J Hum Genet 1998; 6: 552–562.
Maniatis T, Fritsch EF, SambrooK J : Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, 1982.
Greer WL, Lee CL, Callanan MB, Zayed E, Sadek I : Case of acute lymphoblastic leukemia presenting with t(14;18)/BCL2, t(8;14)/cMYC, and t(1;2)/FCGR2B. Am J Hematol 2003; 74: 112–118.
Ishkanian AS, Malloff CA, Watson SK et al: A tiling resolution DNA microarray with complete coverage of the human genome. Nat Genet 2004; 36: 299–303.
Gitschier J, Drayna D, Tuddenham EG, White RL, Lawn RM : Genetic mapping and diagnosis of haemophilia A achieved through a BclI polymorphism in the factor VIII gene. Nature 1985; 314: 738–740.
Rossiter JP, Young M, Kimberland ML et al: Factor VIII gene inversions causing severe hemophilia A originate almost exclusively in male germ cells. Hum Mol Genet 1994; 3: 1035–1039.
Sawecka J, Skulimowska J, Windyga J, Lopaciuk S, Koscielak J : Prevalence of the intron 22 inversion of the factor VIII gene and inhibitor development in Polish patients with severe hemophilia A. Arch Immunol Ther Exp (Warsz) 2005; 53: 352–356.
Xie YG, Zheng H, Leggo J, Scully MF, Lillicrap D : A founder factor VIII mutation, valine 2016 to alanine, in a population with an extraordinarily high prevalence of mild hemophilia A. Thromb Haemost 2002; 87: 178–179.
Bagnall RD, Waseem N, Green PM, Giannelli F : Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood 2002; 99: 168–174.
Williams IJ, Abuzenadah A, Winship PR et al: Precise carrier diagnosis in families with haemophilia A: use of conformation sensitive gel electrophoresis for mutation screening and polymorphism analysis. Thromb Haemost 1998; 79: 723–726.
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW : Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239.
Tilley WD, Marcelli M, Wilson JD, McPhaul MJ : Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA 1989; 86: 327–331.
Siegbahn A, Odlind V, Hedner U, Venge P : Coagulation and fibrinolysis during the normal menstrual cycle. Upsala J Med Sci 1989; 94: 137–152.
Waters JJ, Campbell PL, Crocker AJ, Campbell CM : Phenotypic effects of balanced X-autosome translocations in females: a retrospective survey of 104 cases reported from UK laboratories. Hum Genet 2001; 108: 318–327.
Iversen PO, Groot PD, Hjeltnes N, Andersen TO, Mowinckel MC, Sandset PM : Impaired circadian variations of haemostatic and fibrinolytic parameters in tetraplegia. Br J Haematol 2002; 119: 1011–1016.
Plenge RM, Hendrich BD, Schwartz C et al: A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet 1997; 17: 353–356.
Orstavik KH, Magnus P, Reisner H, Berg K, Graham JB, Nance W : Factor VIII and factor IX in a twin population. Evidence for a major effect of ABO locus on factor VIII level. Am J Hum Genet 1985; 37: 89–101.
Greer WL, Kwong PC, Peacocke M, Ip P, Rubin LA, Siminovitch KA : X-chromosome inactivation in the Wiskott–Aldrich syndrome: a marker for detection of the carrier state and identification of cell lineages expressing the gene defect. Genomics 1989; 4: 60–67.
Tomkins DJ, McDonald HL, Farrell SA, Brown CJ : Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet 2002; 10: 44–51.
West JD, Frels WI, Chapman VM, Papaioannou VE : Preferential expression of the maternally derived X chromosome in the mouse yolk sac. Cell 1977; 12: 873–882.
Sharman GB : Late DNA replication in the paternally derived X chromosome of female kangaroos. Nature 1971; 230: 231–232.
Zeng SM, Yankowitz J : X-inactivation patterns in human embryonic and extra-embryonic tissues. Placenta 2003; 24: 270–275.
Cattanach BM, Isaacson JH : Controlling elements in the mouse X chromosome. Genetics 1967; 57: 331–346.
Chadwick LH, Willard HF : Genetic and parent-of-origin influences on X chromosome choice in Xce heterozygous mice. Mamm Genome 2005; 16: 691–699.
Cau M, Addis M, Congiu R et al: A locus for familial skewed X chromosome inactivation maps to chromosome Xq25 in a family with a female manifesting Lowe syndrome. J Hum Genet 2006; 51: 1030–1036.
Acknowledgements
This work was supported by grants from the CDHA, Halifax, Nova Scotia (WG, SD), and the Canadian Hemophilia Society (WG, SD). NR was supported by scholarships from the Canadian Institutes of Health Research (CIHR), and the Killam Foundation. Part of this work was completed by the Australian Genome Research Facility (AGRF), Victoria, Australia. We acknowledge their contribution and the support that the AGRF receives from the Commonwealth. We thank Dr D Lillicrap and J Leggo at the National Program for Hemophilia Mutation Testing and the AHCDC for their support. We thank Dr R Howell for critical reading of the paper, Dr B Morash for technical guidance, and P Steele for patient recruitment and correspondence.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renault, N., Dyack, S., Dobson, M. et al. Heritable skewed X-chromosome inactivation leads to haemophilia A expression in heterozygous females. Eur J Hum Genet 15, 628–637 (2007). https://doi.org/10.1038/sj.ejhg.5201799
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201799
Keywords
This article is cited by
-
Unfavorable switching of skewed X chromosome inactivation leads to Menkes disease in a female infant
Scientific Reports (2024)
-
A novel quantitative targeted analysis of X-chromosome inactivation (XCI) using nanopore sequencing
Scientific Reports (2023)
-
Severe haemophilia A in a preterm girl with Turner syndrome: case report – a diagnostic and therapeutic challenge for a paediatrician (Part 2)
Italian Journal of Pediatrics (2021)
-
Haemophilia
Nature Reviews Disease Primers (2021)
-
X Chromosome inactivation: a modifier of factor VIII and IX plasma levels and bleeding phenotype in Haemophilia carriers
European Journal of Human Genetics (2021)