Abstract
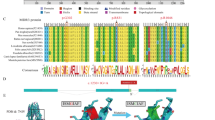
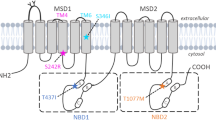
Progressive familial intrahepatic cholestasis type 3 (PFIC3) is an autosomal-recessive disorder due to mutations in the ATP-binding cassette, subfamily B, member 4 gene (ABCB4). ABCB4 is the liver-specific membrane transporter of phosphatidylcholine, a major and exclusive component of mammalian bile. The disease is characterized by early onset of cholestasis with high serum γ-glutamyltranspeptidase activity, which progresses into cirrhosis and liver failure before adulthood. Presently, about 20 distinct ABCB4 mutations associated to PFIC3 have been described. We report the molecular characterization of 68 PFIC3 index cases enrolled in a multicenter study, which represents the largest cohort of PFIC3 patients screened for ABCB4 mutations to date. We observed 31 mutated ABCB4 alleles in 18 index cases with 29 distinct mutations, 25 of which are novel. Despite the lack of structural information on the ABCB4 protein, the elucidation of the three-dimensional structure of bacterial homolog allows the three-dimensional model of ABCB4 to be built by homology modeling and the position of the mutated amino-acids in the protein tertiary structure to be located. In a significant fraction of the cases reported in this study, the mutation should result in substantial impairment of ABCB4 floppase activity. The results of this study provide evidence of the broad allelic heterogeneity of the disease, with causative mutations spread along 14 of the 27 coding exons, but with higher prevalence on exon 17 that, as recently shown for the closely related paralogous ABCB1 gene, could contain an evolutionary marker for mammalian ABCB4 genes in the seventh transmembrane segment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
De Vree JML, Jacquemin E, Sturm E et al: Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 1998; 95: 282–287.
Jacquemin E, De Vree JML, Cresteil D et al: The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 2001; 120: 1448–1458.
Higgins CF : ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992; 8: 67–113.
Van der Bliek AM, Baas F, Ten Houte de Lange T, Kooiman PM, Van der Velde-Koerts T, Borst P : The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J 1987; 6: 3325–3331.
Kast C, Canfield V, Levenson R, Gros P : Membrane topology of P-glycoprotein as determined by epitope insertion: transmembrane organization of the N-terminal domain of mdr3. Biochemistry 1995; 34: 4402–4411.
Kast C, Canfield V, Levenson R, Gros P : Transmembrane organization of mouse P-glycoprotein determined by epitope insertion and immunofluorescence. J Biol Chem 1996; 271: 9240–9248.
Klein I, Sarkadi B, Varadi A : An inventory of the human ABC proteins. Biochim Biophys Acta 1999; 1461: 237–262.
Holland IB, Blight MA : ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol 1999; 293: 381–399.
Dawson RJ, Locher KP : Structure of a bacterial multidrug ABC transporter. Nature 2006; 443: 180–185.
Sankatsing SU, Beijnen JH, Schinkel AH et al: P glycoprotein in human immunodeficiency virus type 1 infection and therapy. Antimicrob Agents Chemother 2004; 48: 1073–1081.
Burk O, Arnold KA, Nussler AK et al: Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol 2005; 67: 1954–1965.
Germann UA, Pastan I, Gottesman MM : P-glycoproteins: mediators of multidrug resistance. Semin Cell Biol 1993; 4: 63–76.
Annilo T, Chen ZQ, Shulenin S et al: Evolution of the vertebrate ABC gene family: Analysis of gene birth and death. Genomics 2006; 88: 1–11.
Oude Elferink RP, Paulusma CC : Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch 2006; 453: 601–610.
Smit JJ, Schinkel AH, Oude Elferink RP et al: Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993; 75: 451–462.
Chen HL, Chang PS, Hsu HC et al: Progressive familial intrahepatic cholestasis with high gamma-glutamyltranspeptidase levels in Taiwanese infants: role of MDR3 gene defect? Pediatr Res 2001; 50: 50–55.
Lincke CR, Smit JJ, van der Velde-Koerts T, Borst P : Structure of the human MDR3 gene and physical mapping of the human MDR locus. J Biol Chem 1991; 266: 5303–5310.
Kopp J, Schwede T : The SWISS-MODEL repository: new features and functionalities. Nucleic Acids Res 2006; 34: D315–D318.
Jones TA, Zou JY, Cowan SW, Kjeldgaard M : Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 1993; 50: 157–163.
Tatusova TA, Madden TL : BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 1999; 174: 247–250.
Shyamala V, Baichwal V, Beall E, Ames GF : Structure–function analysis of the histidine permease and comparison with cystic fibrosis mutations. J Biol Chem 1991; 266: 18714–18719.
Ambudkar SV, Kim IW, Xia D, Sauna ZE : The A-loop, a novel conserved aromatic acid subdomain upstream of the Walker A motif in ABC transporters, is critical for ATP binding. FEBS Lett 2006; 580: 1049–1055.
Urbatsch IL, Julien M, Carrier I, Rousseau ME, Cayrol R, Gros P : Mutational analysis of conserved carboxylate residues in the nucleotide binding sites of P-glycoprotein. Biochemistry 2000; 39: 14138–14149.
Loo TW, Clarke DM : Location of the rhodamine-binding site in the human multidrug resistance P-glycoprotein. J Biol Chem 2002; 277: 44332–44338.
Loo TW, Bartlett MC, Clarke DM : Processing mutations located throughout the human multidrug resistance P-glycoprotein disrupt interactions between the nucleotide binding domains. J Biol Chem 2004; 279: 38395–38401.
Loo TW, Clarke DM : Identification of residues in the drug-binding domain of human P-glycoprotein. J Biol Chem 1999; 274: 35388–35392.
Hrycyna CA, Airan LE, Germann UA, Ambudkar SV, Pastan I, Gottesman MM : Structural flexibility of the linker region of human P-glycoprotein permits ATP hydrolysis and drug transport. Biochemistry 1998; 37: 13660–13673.
Loo TW, Bartlett MC, Clarke DM : Transmembrane segment 7 of human P-glycoprotein forms part of the drug-binding pocket. Biochem J 2006; 399: 351–359.
Acknowledgements
We thank the families of the patients for their collaboration. This study was supported in part by the Italian Ministry of University and Research (MIUR), PRIN contract number 2005068307; grant Regione Lombardia, DG Sanità n. 12298 (2004), DG Sanità n.19081 (2005) and by Eurogentest-Network of Excellence, contract n.o. FP6-512148-2004. We are grateful to the participants in the ‘Multicenter study of Italian Society for Pediatric Gastroenterology, Hepatology and Nutrition’: Prof G Maggiore, Clinica Pediatrica Università di Pisa; Dr G Torre, Centro Trapianto di Fegato, Bergamo; Dr R Iorio, Clinica Pediatrica, Università di Napoli; Dr M Resti, Azienda Ospedaliera Meyer, Firenze; Dr M Calacoci, Clinica Pediatrica, Università di Ferrara; Dr E Castellano (U. O. Pediatria III) and MG Marazzi (U. O. Malattie Infettive), IRCCS G. Gaslini, Genova; Prof F Balli, Clinica Pediatrica, Università di Modena; Prof P Vajro, Dipartimento di Pediatria, Università di Napoli; Dr E Ferretti, ASL 1 Napoli est; Prof L Zancan, Clinica pediatrica, Università di Padova; Dr S Martelossi, Clinica Pediatrica, IRCCS Burlo Garofalo, Trieste; Dr F Oliveri, U.O. Gastroenterologia ed Epatologia, Ospedale S Chiara, Pisa. We are grateful to Dr. Giandomenico Russo for oligonucleotides sequences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Degiorgio, D., Colombo, C., Seia, M. et al. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3). Eur J Hum Genet 15, 1230–1238 (2007). https://doi.org/10.1038/sj.ejhg.5201908
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201908
Keywords
This article is cited by
-
Rare variant contribution to cholestatic liver disease in a South Asian population in the United Kingdom
Scientific Reports (2023)
-
Clinical and genetic characterization of pediatric patients with progressive familial intrahepatic cholestasis type 3 (PFIC3): identification of 14 novel ABCB4 variants and review of the literatures
Orphanet Journal of Rare Diseases (2022)
-
Case report: progressive familial intrahepatic cholestasis type 3 with compound heterozygous ABCB4 variants diagnosed 15 years after liver transplantation
BMC Medical Genetics (2020)
-
Cryptogenic cholestasis in young and adults: ATP8B1, ABCB11, ABCB4, and TJP2 gene variants analysis by high-throughput sequencing
Journal of Gastroenterology (2018)
-
Klinische Genetik der Gallenwegserkrankungen
Der Gastroenterologe (2017)