Abstract
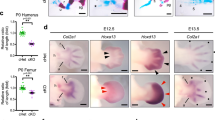
Urogenital birth defects are one of the common phenotypes observed in hereditary human disorders. In particular, limb malformations are often associated with urogenital developmental abnormalities, as the case for Hand–foot–genital syndrome displaying similar hypoplasia/agenesis of limbs and external genitalia. Split-hand/split-foot malformation (SHFM) is a syndromic limb disorder affecting the central rays of the autopod with median clefts of the hands and feet, missing central fingers and often fusion of the remaining ones. SHFM type 1 (SHFM1) is linked to genomic deletions or rearrangements, which includes the distal-less-related homeogenes DLX5 and DLX6 as well as DSS1. SHFM type 4 (SHFM4) is associated with mutations in p63, which encodes a p53-related transcription factor. To understand that SHFM is associated with urogenital birth defects, we performed gene expression analysis and gene knockout mouse model analyses. We show here that Dlx5, Dlx6, p63 and Bmp7, one of the p63 downstream candidate genes, are all expressed in the developing urethral plate (UP) and that targeted inactivation of these genes in the mouse results in UP defects leading to abnormal urethra formation. These results suggested that different set of transcription factors and growth factor genes play similar developmental functions during embryonic urethra formation. Human SHFM syndromes display multiple phenotypes with variations in addition to split hand foot limb phenotype. These results suggest that different genes associated with human SHFM could also be involved in the aetiogenesis of hypospadias pointing toward a common molecular origin of these congenital malformations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yamada G, Satoh Y, Baskin LS, Cunha GR : Cellular and molecular mechanisms of development of the external genitalia. Differentiation 2003; 71: 445–460.
Yamada G, Suzuki K, Haraguchi R et al: Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn 2006; 235: 1738–1752.
Huang WW, Yin Y, Bi Q et al: Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol 2005; 19: 669–682.
Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR : Urethral seam formation and hypospadias. Cell Tissue Res 2001; 305: 379–387.
Haraguchi R, Mo R, Hui C et al: Unique functions of Sonic hedgehog signaling during external genitalia development. Development 2001; 128: 4241–4250.
Haraguchi R, Suzuki K, Murakami R et al: Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 2000; 127: 2471–2479.
Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ : Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol 2002; 247: 26–46.
Suzuki K, Bachiller D, Chen YP et al: Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development 2003; 130: 6209–6220.
Yamaguchi TP, Bradley A, McMahon AP, Jones S : A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126: 1211–1223.
Morgan EA, Nguyen SB, Scott V, Stadler HS : Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 2003; 130: 3095–3109.
Kurzrock EA, Baskin LS, Cunha GR : Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation 1999; 64: 115–122.
Kurzrock EA, Baskin LS, Li Y, Cunha GR : Epithelial–mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs 1999; 164: 125–130.
Yang A, Schweitzer R, Sun D et al: p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999; 398: 714–718.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A : p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713.
Basel D, Kilpatrick MW, Tsipouras P : The expanding panorama of split hand foot malformation. Am J Med Genet A 2006; 140: 1359–1365.
Simeone A, Acampora D, Pannese M et al: Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci USA 1994; 91: 2250–2254.
Crackower MA, Scherer SW, Rommens JM et al: Characterization of the split hand/split foot malformation locus SHFM1 at 7q21.3–q22.1 and analysis of a candidate gene for its expression during limb development. Hum Mol Genet 1996; 5: 571–579.
Merlo GR, Paleari L, Mantero S et al: Mouse model of split hand/foot malformation type I. Genesis 2002; 33: 97–101.
Robledo RF, Rajan L, Li X, Lufkin T : The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev 2002; 16: 1089–1101.
Panganiban G : Distal-less function during Drosophila appendage and sense organ development. Dev Dyn 2000; 218: 554–562.
Merlo GR, Zerega B, Paleari L, Trombino S, Mantero S, Levi G : Multiple functions of Dlx genes. Int J Dev Biol 2000; 44: 619–626.
Zerucha T, Ekker M : Distal-less-related homeobox genes of vertebrates: evolution, function, and regulation. Biochem Cell Biol 2000; 78: 593–601.
Ghanem N, Jarinova O, Amores A et al: Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res 2003; 13: 533–543.
Stock DW, Ellies DL, Zhao Z, Ekker M, Ruddle FH, Weiss KM : The evolution of the vertebrate Dlx gene family. Proc Natl Acad Sci USA 1996; 93: 10858–10863.
Park BK, Sperber SM, Choudhury A et al: Intergenic enhancers with distinct activities regulate Dlx gene expression in the mesenchyme of the branchial arches. Dev Biol 2004; 268: 532–545.
Ianakiev P, Kilpatrick MW, Toudjarska I, Basel D, Beighton P, Tsipouras P : Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet 2000; 67: 59–66.
Godin RE, Takaesu NT, Robertson EJ, Dudley AT : Regulation of BMP7 expression during kidney development. Development 1998; 125: 3473–3482.
Zeisberg M, Hanai J, Sugimoto H et al: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003; 9: 964–968.
Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I : p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development 2006; 133: 1553–1563.
Beverdam A, Merlo GR, Paleari L et al: Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis 2002; 34: 221–227.
Temtamy SA, McKusick VA : The genetics of hand malformations. Birth Defects Orig Artic Ser 1978; 14 (i–xviii): 1–619.
Faiyaz ul Haque M, Uhlhaas S, Knapp M et al: Mapping of the gene for X-chromosomal split-hand/split-foot anomaly to Xq26–q26.1. Hum Genet 1993; 91: 17–19.
Nunes ME, Schutt G, Kapur RP et al: A second autosomal split hand/split foot locus maps to chromosome 10q24–q25. Hum Mol Genet 1995; 4: 2165–2170.
Del Campo M, Jones MC, Veraksa AN et al: Monodactylous limbs and abnormal genitalia are associated with hemizygosity for the human 2q31 region that includes the HOXD cluster. Am J Hum Genet 1999; 65: 104–110.
O'Quinn JR, Hennekam RC, Jorde LB, Bamshad M : Syndromic ectrodactyly with severe limb, ectodermal, urogenital, and palatal defects maps to chromosome 19. Am J Hum Genet 1998; 62: 130–135.
Giltay JC, Wittebol-Post D, van Bokhoven H, Kastrop PM, Lock MT : Split hand/split foot, iris/choroid coloboma, hypospadias and subfertility: a new developmental malformation syndrome? Clin Dysmorphol 2002; 11: 231–235.
Garcia-Ortiz JE, Banda-Espinoza F, Zenteno JC, Galvan-Uriarte LM, Ruiz-Flores P, Garcia-Cruz D : Split hand malformation, hypospadias, microphthalmia, distinctive face and short stature in two brothers suggest a new syndrome. Am J Med Genet A 2005; 135: 21–27.
Elliott AM, Evans JA, Chudley AE : Split hand foot malformation (SHFM). Clin Genet 2005; 68: 501–505.
Baskin LS (ed.): Hypospadias and genital development. Adv Exp Med Biol 2004 Philadelphia: Kluwer Academic/Plenum Publication.
Baskin L : Hypospadias: a critical analysis of cosmetic outcomes using photography. BJU Int 2001; 87: 534–539.
Beleza-Meireles A, Lundberg F, Lagerstedt K et al: FGFR2, FGF8, FGF10 and BMP7 as candidate genes for hypospadias. Eur J Hum Genet 2007; 15: 405–410.
Chen T, Li Q, Xu J et al: Mutation screening of BMP4, BMP7, HOXA4 and HOXB6 genes in Chinese patients with hypospadias. Eur J Hum Genet 2007; 15: 23–28.
Corcoran JP, Ferretti P : Keratin 8 and 18 expression in mesenchymal progenitor cells of regenerating limbs is associated with cell proliferation and differentiation. Dev Dyn 1997; 210: 355–370.
Casanova ML, Bravo A, Ramirez A et al: Exocrine pancreatic disorders in transsgenic mice expressing human keratin 8. J Clin Invest 1999; 103: 1587–1595.
Paramio JM, Segrelles C, Ruiz S, Jorcano JL : Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol 2001; 21: 7449–7459.
Penington EC, Hutson JM : The urethral plate – does it grow into the genital tubercle or within it? BJU Int 2002; 89: 733–739.
Wei CL, Wu Q, Vega VB : A global map of p53 transcription-factor binding sites in the human genome. Cell 2006; 124: 207–219.
Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR : p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 2004; 18: 126–131.
Vaahtokari A, Aberg T, Jernvall J, Keranen S, Thesleff I : The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 1996; 54: 39–43.
Satoh Y, Haraguchi R, Wright TJ et al: Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat Embryol (Berlin) 2004; 208: 479–486.
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G : BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 1995; 9: 2808–2820.
Dudley AT, Lyons KM, Robertson EJ : A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 1995; 9: 2795–2807.
Acknowledgements
Invaluable support from Liz Robertson is appreciated. We thank Drs Giorgio R Merlo, Alex Joyner, Hisayo Nishida, Mingjun Sun, Shigeaki Kato, Denis Duboule, Chi-Chung Hui, Gail Martin, John McLachlan, Anne M Moon, Sawako Fujikawa, Kenta Sumiyama, Yoshihiko Satoh and Yukiko Ogino for encouragement and helps. We express our appreciation to Shiho Kitagawa for her valuable assistance. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas; General promotion of Cancer research in Japan, by a Grant-in-Aid for Scientific Research on Priority Areas; Mechanisms of Sex Differentiation, by the Global COE Research Program and by a Grant for Child Health and Development (17-2) from the Ministry of Health, Labour and Welfare. GL is supported by the grant ‘GENDACTYL’ of the French Agence National pour la Recherche (ANR). OB is supported by the Telethon (Italy) grant GP0218Y01.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Suzuki, K., Haraguchi, R., Ogata, T. et al. Abnormal urethra formation in mouse models of Split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet 16, 36–44 (2008). https://doi.org/10.1038/sj.ejhg.5201925
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201925
Keywords
This article is cited by
-
Regulation of masculinization: androgen signalling for external genitalia development
Nature Reviews Urology (2018)
-
Anorectal malformation associated with a mutation in the P63 gene in a family with split hand–foot malformation
International Journal of Colorectal Disease (2013)
-
The α/β carboxy-terminal domains of p63 are required for skin and limb development. New insights from the Brdm2 mouse which is not a complete p63 knockout but expresses p63 γ-like proteins
Cell Death & Differentiation (2009)