Abstract
Data sources
Searches for appropriate studies were made using the following: Cochrane Neuromuscular Disease Group Register, Medline, Embase and LILACS (Latin American and Caribbean Literature on the Health Sciences) together the Chinese Biomedical Retrieval System, the database of the Chinese Cochrane Centre, conference paper databases and checked bibliographies. 10 Chinese journals were searched by hand.
Study selection
Randomised controlled trials (RCT) or quasi-randomised controlled trials were included.
Data extraction and synthesis
Two authors decided which trials fitted the inclusion criteria and graded methodological quality independently.
Results
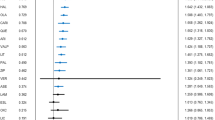
Nine trials of different non-antiepileptic drugs involving 223 participants were included. Each trial investigated one non-antiepileptic drug.
Two trials tested baclofen. In one, more people achieved 50% reduction from baseline than with placebo [relative risk (RR), 15.00; 95% confidence interval (CI), 0.97–231.84; P 0.05]. In the other, slightly more participants who took baclofen showed a 75% reduction in attacks on the tenth day compared with carbamazepine (RR, 2.38; 95% CI, 0.83–6.85; P 0.11). One trial showed no significant difference in reduction in average daily frequency of attacks with Baclofen compared with Racemic Baclofen.
Tizanidine was investigated in two trials. In one, the proportion of people with a reduction in the mean number of paroxysms per day increased with tizanidine compared with placebo (RR, 8.00; 95% CI, 1.21–52.69; P 0.03). In the other, one of five participants improved their visual analogue scale score with tizanidine and four of six did so having taken carbamazepine (RR, 0.30; 95% CI, 0.05–1.89; P 0.20).
One study showed that the improvement in mean values of pain scores with tocainide was similar to that of carbamazepine. In a further trial, more participants improved during the pimozide than the carbamazepine period (RR, 1.78; 95% CI, 1.39–2.28). In another, a 0.5% instillation of proparacaine hydrochloride into the eyes did not produce significantly different results from placebo (RR, 1.06; 95% CI, 0.37–2.99; P 0.92). Finally, there was moderate or marked improvement in a study in seven of nine participants who took clomipramine and three of nine who took amitriptyline during a 12-week treatment (RR, 2.33; 95% CI, 0.87–6.27).
Conclusions
Trials of non-antiepileptic drugs for treating trigeminal neuralgia have all been limited by poor methodological quality or poor reporting. There is insufficient evidence from randomised clinical trials to show significant benefit from non-antiepileptic drugs for trigeminal neuralgia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A . Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev 2005; issue number 3.
Wiffen PJ, McQuay HJ, Moore RA . Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev 2005; issue number 3.
Wiffen PJ, McQuay HJ, Edwards JE, Moore RA . Gabapentin for acute and chronic pain. Cochrane Database Syst Rev 2005; issue number 3.
Dworkin RH, Turk DC, Farrar JT . et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Luisa M Fernandez Mauleffinch, Cochrane Oral Health Group, MANDEC, School of Dentistry, University of Manchester, Higher Cambridge Street, Manchester M15 6FH, UK.
He L, Wu B, Zhou M. Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database of Systematic Reviews 2006; issue 3
Rights and permissions
About this article
Cite this article
Zakrzewska, J. Robust randomised control trials needed for drug treatments for trigeminal neuralgia. Evid Based Dent 7, 107 (2006). https://doi.org/10.1038/sj.ebd.6400463
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ebd.6400463