Summary
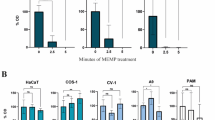
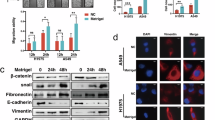
We isolated six clones of weakly tumorigenic fibrosarcoma (QR) from the tumorigenic clone BMT-11 cl-9. The QR clones were unable to grow in normal C57BL/6 mice when injected s.c. (1 × 105 cells). However, they formed aggressive tumours upon co-implantation with a ‘foreign body’, i.e. a gelatin sponge, and the rate of tumour take ranged from 8% to 58% among QR clones. The enhanced tumorigenicity was due to host cell-mediated reaction to the gelatin sponge (inflammation). Immunoblot analysis and enzyme activity assay revealed a significant inverse correlation between the frequencies of tumour formation by QR clones and the levels of manganese superoxide dismutase (Mn-SOD, P<0.005) and glutathione peroxidase (GPχ, P<0.01) in the respective tumour clones. Electron spin resonance (ESR) revealed that superoxide-scavenging ability of cell lysates of the QR clone with high level of Mn-SOD was significantly higher than that with low level of the antioxidative enzyme in the presence of potassium cyanide, an inhibitor for copper–zinc superoxide dismutase (CuZn-SOD) (P<0.001). Minisatellite mutation (MSM) induced by the inflammatory cells in tumour cells were investigated by DNA fingerprint analysis after QR clones had been co-cultured with gelatin-sponge-reactive cells. The MSM rate was significantly higher in the subclones with low levels of Mn-SOD and GPχ (P<0.05) than in the subclones with high levels of both enzymes. The MSM of the subclones with low levels of both enzymes was inhibited in the presence of mannitol, a hydroxyl radical scavenger. The content of 8-hydroxydeoxyguanosine (8-OHdG) by which the cellular DNA damage caused by active oxygen species can be assessed was significantly low in the tumours arising from the QR clone with high levels of Mn-SOD and GPχ even if the clone had been co-implanted with gelatin sponge, compared with the arising tumour from the QR clone with low levels of those antioxidative enzymes (P<0.001). In contrast, CuZn-SOD and catalase levels in the six QR clones did not have any correlation with tumour progression parameters. These results suggest that tumour progression is accelerated by inflammation-induced active oxygen species particularly accompanied with declined levels of intracellular antioxidative enzymes in tumour cells.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Akporiaye, E. T. & Kudalore, M. K. (1989). Implantation of a gelatin sponge as a model for effector recruitment: tumor growth inhibition by T-lymphocytes recovered from a site of tumour rejection. Cancer Immunol Immunother 29: 199–204.
Ames, B. N., Shigenaga, M. K. & Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922.
Aruoma, O. I., Halliwell, B., Gajewski, E. & Dizdaroglu, M. (1991). Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 273: 601–604.
Ascher, N. A., Eerguson, R. M., Hoffman, R. & Simmons, R. L. (1979). Partial characterization of cytotoxic cells infiltrating spongematrix allografts. Transplantation 27: 254–259.
Badway, J. A. & Karnovsky, M. L. (1980). Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem 49: 695–726.
Beauchamp, C. & Fridovich, I. (1971). Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287.
Beers, Jr R. F. & Sizer, I. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195: 133–140.
Beutler, E. (1984). A manual of biochemical methods. In Red Cell Metabolism, edn. 3 Beutler E (ed.) pp. 72–136, Grune & Stratton: Philadelphia
Blackburn, E. H. (1991). Structure and function of telomeres. Nature 350: 569–573.
Chance, B., Sies, H. & Boveris, A. (1979). Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605.
Choi, P. M. & Zelig, M. P. (1994). Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 35: 950–954.
Cohen, G. & Hochstein, P. (1963). Glutathione peroxidase: the primary agent for the elimination of H2O2 in erythrocytes. Biochemistry 2: 1420–1428.
Cohen, G., Dembiec, D. & Marcus, J. (1970). Measurement of catalase activity in tissue extracts. Anal Biochem 34: 30–38.
Filmus, J. & Kerbel, R. S. (1993). Development of resistance mechanisms to the growth-inhibitory effects of transforming growth factor-beta during tumor progression. Curr Opin Oncol 5: 123–129.
Flóhe, L. (1982). Glutathione peroxidase brought into focus. In Free Radicals in Biology, Pryor WA (ed.), pp. 223–254, Academic Press: New York
Foulds, L. (1965). Multiple etiologic factors in neoplastic development. Cancer Res 25: 1339–1347.
Halliwell, B. (1987). Oxidants and human disease: some new concepts. FASEB J 1: 358–364.
Hastie, N. D., Dempster, M., Dunlop, M. G., Thompson, A. M., Green, D. K. & Allshire, R. C. (1990). Telomere reduction in human colorectal carcinoma and with aging. Nature 346: 866–868.
Heppner, G. & Dorcey, L. (1988). Macrophages and development of cancer. In Macrophages and Cancer, Heppner GH, Fulton AM (eds.), pp. 197–208, CRC Press: Boca Raton, Florida
Ischiropoulos, H., Zhu, L. & Beckman, J. S. (1992). Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys 298: 446–451.
Ishikawa, M., Hosokawa, M., Oh-hara, N., Niho, Y. & Kobayashi, H. (1987a). Marked granulocytosis in C57Bl/6 mice bearing a transplanted BMT-11 fibrosarcoma. J Natl Cancer Inst 78: 567–571.
Ishikawa, M., Okada, F., Hamada, J-I, Hosokawa, M. & Kobayashi, H. (1987b). Changes in the tumorigenic and metastatic properties of tumor cells treated with quercetin or 5-azacytidine. Int J Cancer 39: 338–342.
Jeffreys, A. J., Wilson, V. & Thein, S. L. (1985). Hypervariable ‘minisatellite’ regions in human DNA. Nature 314: 67–73.
Kasai, H. & Nishimura, S. (1984). Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res 12: 2137–2145.
Kasai, H., Nishimura, S., Kurokawa, Y. & Hayashi, Y. (1987). Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis 8: 1959–1961.
Kawaguchi, T., Noji, S., Uda, T., Nakashima, Y., Takeyasu, A., Kawai, Y., Takagi, H., Tohyama, M. & Taniguchi, N. (1989). A monoclonal antibody against COOH– terminal peptide of human liver manganese superoxide dismutase. J Biol Chem 264: 5762–5767.
Kayanoki, Y., Fujii, J., Suzuki, K., Kawata, S., Matsuzawa, Y. & Taniguchi, N. (1994). Suppression of antioxidative enzyme expression by transforming growth factor-β1 in rat hepatocytes. J Biol Chem 269: 15488–15492.
Kitazawa, T., Kominami, R., Tanaka, R., Wakabayashi, K. & Nagao, M. (1994). 2-Hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine induction of recombinational mutations in mammalian cell lines as detected by DNA fingerprinting. Mol Carcinog 9: 67–70.
Kuwabara, M., Inukai, N., Inanami, O., Miyake, Y. I., Tsunoda, N., Maki, Y. & Sato, F. (1996). Lipid peroxide levels and superoxide-scavenging abilities of sera obtained from hotbred (thoroughbred) horses. J Vet Med Sci 58: 97–101.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Ledwith, B. J., Storer, R. D., Prahalada, S., Manam, S., Leander, K. R., van Zwieten, M. J., Nichols, W. W. & Bradley, M. O. (1990). DNA fingerprinting of 7,12-dimethylbenz[a]anthracene-induced and spontaneous CD-1 mouse liver tumors. Cancer Res 50: 5245–5249.
Ledwith, B. J., Joslyn, D. J., Troilo, P., Leander, K. R., Clair, J. H., Soper, K. A., Manam, S., Prahalada, S., van Zwieten, M. J. & Nichols, W. W. (1995). Induction of minisatellite DNA rearrangements by genotoxic carcinogens in mouse liver tumors. Carcinogenesis 16: 1167–1172.
Leek, R. D., Harris, A. L. & Lewis, C. E. (1994). Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukocyte Biol 56: 423–435.
Lewis, J. G. & Adams, D. O. (1987). Inflammation, oxidative DNA damage, and carcinogenesis. Environ Health Perspect 76: 19–27.
Li, J-J, Oberley, L. W., St Clair, D. K., Ridnour, L. A. & Oberley, T. D. (1995). Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene 10: 1989–2000.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275.
Matsumura, Y. & Tarin, D. (1992). DNA fingerprinting survey of various human tumors and their metastases. Cancer Res 52: 2174–2179.
Middleton, M. M. & Campbell, P. A. (1989). Functions of purified mouse neutrophils isolated from gelatin sponges. J Leukocyte Biol 46: 461–466.
Mitani, K., Takahashi, Y. & Kominami, R. (1990). A GGCAGG motif in minisatellites affecting their germline instability. J Biol Chem 265: 15203–15210.
Nagayasu, H., Hamada, J-I, Nakata, D., Shibata, T., Kobayashi, M., Hosokawa, M. & Takeichi, N. (1998). Reversible and irreversible tumor progression of a weakly malignant rat mammary carcinoma cell line by in vitro exposure to epidermal growth factor. Int J Oncol 12: 197–202.
Nakae, D., Mizumoto, Y., Kobayashi, E., Noguchi, O. & Konishi, Y. (1995). Improved genomic/nuclear DNA extraction for 8-hydroxydeoxyguanosine analysis of small amounts of rat liver tissue. Cancer Lett 97: 233–239.
Nakagawa, H., Kaneko, S., Shima, H., Inamori, H., Fukuda, H., Kominami, R., Sugimura, T. & Nagao, M. (1997). Induction of minisatellite mutation in NIH3T3 cells by treatment with the tumor promoter okadaic acid. Proc Natl Acad Sci USA 94: 10813–10816.
Oberley, T. D. & Oberley, L. W. (1997). Antioxidant enzyme levels in cancer. Histol Histopathol 12: 525–535.
Okada, F., Hosokawa, M., Hasegawa, J., Ishikawa, M., Chiba, I., Nakamura, Y. & Kobayashi, H. (1990). Regression mechanisms of mouse fibrosarcoma cells after in vitro exposure to quercetin: diminution of tumorigenicity with a corresponding decrease in the production of prostaglandin E2. Cancer Immunol Immunother 31: 358–364.
Okada, F., Hosokawa, M., Hamada, J-I, Hasegawa, J., Kato, M., Mizutani, M., Ren, J., Takeichi, N. & Kobayashi, H. (1992). Malignant progression of a mouse fibrosarcoma by host cells reactive to a foreign body (gelatin sponge). Br J Cancer 66: 635–639.
Okada, F., Hosokawa, M., Hamada, J-I, Hasegawa, J., Mizutani, M., Takeichi, N. & Kobayashi, H. (1993). Progression of a weakly tumorigenic mouse fibrosarcoma at the site of early phase of inflammation caused by plastic plates. Jpn J Cancer Res 84: 1230–1236.
Okada, F., Hosokawa, M., Hasegawa, J., Kuramitsu, Y., Nakai, K., Yuan, L., Lao, H., Kobayashi, H. & Takeichi, N. (1994). Enhancement of in vitro prostaglandin E2 production by mouse fibrosarcoma cells after co-culture with various anti-tumour effector cells. Br J Cancer 70: 233–238.
Pitot, H. C. (1986). The natural history of neoplastic development: progression. In Fundamentals of Oncology, Pitot HC (ed.), pp. 163–200, Marcel Dekker: New York
Pitot, H. C. (1989). Progression: the terminal stage in carcinogenesis. Jpn J Cancer Res 80: 599–607.
Rosin, M. P., Anwar, W. A. & Ward, A. J. (1994). Inflammation, chromosomal instability, and cancer: the schistosomiasis model. Cancer Res 54: 1929s–1933s.
Schraufstatter, I., Hyslop, P. A., Jackson, J. H. & Cochrane, C. G. (1988). Oxidant-induced DNA damage of targent cells. J Clin Invest 82: 1040–1050.
Schreck, R. & Baeuerle, P. A. (1991). A role for oxygen radicals as second messengers. Trends Cell Biol 1: 39–42.
Shacter, E., Beecham, E. J., Covey, J. M., Kohn, K. W. & Potter, M. (1988). Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis 9: 2297–2304.
Shibutani, S., Takeshita, M. & Grollman, A. P. (1991). Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349: 431–434.
Shimoda, R., Nagashima, M., Sakamoto, M., Yamaguchi, N., Hirohashim, S., Yokota, J. & Kasai, H. (1994). Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res 54: 5171–5172.
Southern, E. M. (1975). Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–515.
Suemizu, H., Yoshimura, S., Takeichi, N. & Moriuchi, T. (1994). Decreased expression of liver glutathione peroxidase in Long–Evans Cinnamon mutant rats predisposed to hepatitis and hepatoma. Hepatology 19: 694–700.
Suzuki, K., Nakata, T., Seo, H. G., Miyazawa, N., Sugiyama, T. & Taniguchi, N. (1991a). Differential expression of Mn- and Cu,Zn-superoxide dismutases in various tissues of LEC rats. In The LEC Rat, Mori M, Yoshida MC, Takeichi N and Taniguchi N (eds.), pp. 142–148, Springer-Verlag: Tokyo
Suzuki, S., Takada, T., Sugawara, Y., Muto, T. & Kominami, R. (1991b). Quercetin induces recombinational mutations in cultured cells as detected by DNA fingerprinting. Jpn J Cancer Res 82: 1061–1064.
Tabor, E. & Kobayashi, K. (1992). Hepatitis C virus, a causative infectious agent of non-A, non-B hepatitis: prevalence and structure-summary of a conference on hepatitis C virus as a cause of hepatocellular carcinoma. J Natl Cancer Inst 84: 86–90.
Takada, T., Suzuki, S., Sugawara, Y., Kominami, R., Arakawa, M., Niwa, O. & Yokoro, K. (1992). Somatic mutation during metastasis of a mouse fibrosarcoma line detected by DNA fingerprint analysis. Jpn J Cancer Res 83: 165–170.
Taniguchi, N. (1992). Clinical significances of superoxide dismutases: changes in aging, diabetes, ischemia, and cancer. Adv Clin Chem 29: 1–59.
Thein, S. L., Jeffreys, A. J., Gooi, H. C., Cotter, F., Klint, J., O’Cornor, N. T. J., Weatherall, D. J. & Wainscoat, J. S. (1987). Detection of somatic changes in human cancer DNA by DNA fingerprint analysis. Br J Cancer 55: 353–356.
Weitzman, S. A. & Gordon, L. I. (1990). Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood 76: 655–663.
Willard, H. F. (1990). Centromeres of mammalian chromosomes. Trends Genet 6: 410–416.
Yamashina, K., Miller, B. E. & Heppner, G. H. (1986). Macrophage-mediated induction of drug-resistant variants in a mouse mammary tumor cell line. Cancer Res 46: 2396–2401.
Yoshimura, S., Komatsu, N. & Watanabe, K. (1980). Purification and immunohistochemical localization of rat liver glutathione peroxidase. Biochim Biophys Acta 621: 130–137.
Young, M. R. I., Okada, F., Tada, M., Hosokawa, M. & Kobayashi, H. (1991). Association of increased tumor cell responsiveness to prostaglandin E2 with more aggressive tumor behavior. Invasion Metastasis 11: 48–57.
Yu, M. W., You, S. L., Chang, A. S., Lu, S. N., Liaw, Y. F. & Chen, C. J. (1991). Association between hepatitis C virus antibodies and hepatocellular carcinoma in Taiwan. Cancer Res 51: 5621–5625.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Okada, F., Nakai, K., Kobayashi, T. et al. Inflammatory cell-mediated tumour progression and minisatellite mutation correlate with the decrease of antioxidative enzymes in murine fibrosarcoma cells. Br J Cancer 79, 377–385 (1999). https://doi.org/10.1038/sj.bjc.6690060
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bjc.6690060
Keywords
This article is cited by
-
Nrf2-Keap1 Signaling as a Potential Target for Chemoprevention of Inflammation-Associated Carcinogenesis
Pharmaceutical Research (2010)
-
Prevention of inflammation-mediated acquisition of metastatic properties of benign mouse fibrosarcoma cells by administration of an orally available superoxide dismutase
British Journal of Cancer (2006)
-
Conversion of Human Colonic Adenoma Cells to Adenocarcinoma Cells Through Inflammation in Nude Mice
Laboratory Investigation (2000)