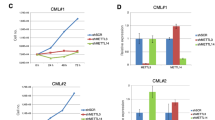
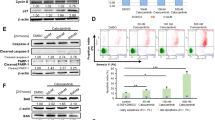
Summary
The translocation (8;21)(q22;q22) is a karyotypic abnormality detected in acute myeloid leukaemia (AML) M2 and results in the formation of the chimeric fusion gene AML1/MTG8. We previously reported that two hammerhead ribozymes against AML1/MTG8 cleave this fusion transcript and also inhibit the proliferation of myeloid leukaemia cell line Kasumi-1 which possesses t(8;21)(q22;q22). In this study, we investigated the mechanisms of inhibition of proliferation in myeloid leukaemic cells with t(8;21)(q22;q22) by ribozymes. These ribozymes specifically inhibited the growth of Kasumi-1 cells, but did not affect the leukaemic cells without t(8;21)(q22;q22). We observed the morphological changes including chromatin condensation, fragmentation and the formation of apoptotic bodies in Kasumi-1 cells incubated with ribozymes for 7 days. In addition, DNA ladder formation was also detected after incubation with ribozymes which suggested the induction of apoptosis in Kasumi-1 cells by the AML1/MTG8 ribozymes. However, the ribozymes did not induce the expression of CD11b and CD14 antigens in Kasumi-1 cells. The above data suggest that these ribozymes therefore inhibit the growth of myeloid leukaemic cells with t(8;21)(q22;q22) by the induction of apoptosis, but not differentiation. We conclude therefore that the ribozymes targeted against AML1/MTG8 may have therapeutic potential for patients with AML carrying t(8;21)(q22;q22) while, in addition, the product of the chimeric gene is responsible for the pathogenesis of myeloid leukaemia.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Asou, H., Tashiro, S., Hamamoto, K., Otsuji, A., Kita, K. & Kamada, N. (1991). Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood 77: 2031–2036.
Cameron, S., Taylor, D. S., TePas, E. C., Speck, N. A. & Mathey-Prevot, B. (1994). Identification of a critical regulatory site in the human interleukin-3 promoter by in vivo footprinting. Blood 83: 2851–2859.
Erickson, P., Gao, J., Chang, K-S, Look, T., Whisenant, E., Raimondi, S., Lasher, R., Trujillo, J., Rowley, J. & Drabkin, H. (1992). Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood 80: 1825–1831.
Frank, R., Zhang, J., Uchida, H., Meyers, S., Hiebert, S. W. & Nimer, S. D. (1995). The AML1/ETO fusion protein blocks transactivation of the GM-CSF promoter by AML1B. Oncogene 11: 2667–2674.
Haseloff, J. & Gerlach, W. L. (1988). Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature 334: 585–591.
Homann, M., Tzortzakaki, S., Rittner, K., Sczakiel, G. & Tabler, M. (1993). Incorporation of the catalytic domain of a hammerhead ribozyme into antisense RNA enhances its inhibitory effect on the replication of human immunodeficiency virus type 1. Nucleic Acids Res 21: 2809–2814.
Kizaki, M., Matsushita, H., Takayama, N., Muto, A., Ueno, H., Awaya, N., Kawai, Y., Asou, H., Kamada, N. & Ikeda, Y. (1996). Establishment and characterization of a novel acute promyelocytic leukemia cell line (UF-1) with retinoic acid-resistant features. Blood 88: 1824–1833.
Klampfer, L., Zhang, J., Zelenetz, A. O., Uchida, H. & Nimer, S. D. (1996). The AML1/ETO fusion protein activates transcription of Bcl-2. Proc Natl Acad Sci USA 93: 14059–14064.
Koeffler, H. P. (1987). Syndromes of acute nonlymphocytic leukemia. Ann Intern Med 107: 748–758.
Koizumi, M., Iwai, S. & Ohtsuka, E. (1988). Construction of a series of several self-cleaving RNA duplexes using synthetic 21-mer. FEBS Lett 228: 228–230.
Kozu, T., Sueoka, E., Okabe, S., Sueoka, N., Komori, A. & Fujiki, H. (1996). Designing of chimeric DNA/RNA hammerhead ribozymes to be targeted against AML1/MTG8 mRNA. J Cancer Res Clin Oncol 122: 254–256.
Lange, W., Cantin, E. M., Finke, J. & Dolken, G. (1993). In vitro and in vivo effects of synthetic ribozymes targeted against BCR/ABL mRNA. Leukemia 7: 1786–1794.
Lanotte, M., Martin-Thouvenin, V., Najman, S., Balerini, P., Valensi, F. & Berger, R. (1991). NB4, a maturation inducible cell line with t(15;17) marker isolated from human acute promyelocytic leukemia (M3). Blood 77: 1080–1086.
Lenny, N., Meyers, S. & Hiebert, S. W. (1995). Functional domains of t(8;21) fusion protein, AML-1/MTG8. Oncogene 11: 1761–1769.
Leopold, L. H., Shore, S. K., Newkirk, T. A., Reddy, R. M. V. & Reddy, E. P. (1995). Multi-unit ribozyme-mediated cleavage of bcr-abl mRNA in myeloid leukemia. Blood 85: 2162–2170.
Matsushita, H., Kobayashi, H., Mori, S., Kizaki, M. & Ikeda, Y. (1995). Ribozymes cleave the AML1/MTG8 fusion transcript and inhibit proliferation of leukemic cells with t(8;21). Biochem Biophys Res Commun 215: 431–437.
Meyers, S., Downing, J. R. & Hiebert, S. W. (1993). Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein–protein interactions. Mol Cell Biol 13: 6336–6345.
Meyers, S., Lenny, N. & Hiebert, S. W. (1995). The t(8;21) fusion protein interferes with AML-1B-1 dependent transcriptional activation. Mol Cell Biol 15: 1974–1982.
Miyoshi, H., Kozu, T., Shimizu, K., Enomoto, K., Maseki, N., Kaneko, Y., Kamada, N. & Ohki, M. (1993). The t(8;21)translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J 12: 2715–2721.
Miyoshi, H., Ohira, M., Shimizu, K., Mitani, K., Hirai, H., Imai, T., Yokoyama, K. & Ohki, M. (1995). Alternative splicing and genomic structure of the AML1 gene involved in acute myeloid leukemia. Nucleic Acid Res 23: 2762–2769.
Muto, A., Mori, S., Matsushita, H., Awaya, N., Ueno, H., Takayama, N., Okamoto, S., Kizaki, M. & Ikeda, Y. (1996). Serial quantification of minimal residual disease of t(8;21) acute myelogenous leukemia with RT-competitive PCR assay. Br J Haematol 95: 85–94.
Nuchprayoon, I., Meyers, S., Scott, L. M., Suzow, J., Hiebert, S. & Friedman, A. D. (1994). PEBP2/CBF, the murine homologue of the human myeloid AML1 and PEBP2β/CBFβ proto-oncoproteins, regulates the murine myeloperoxidase and neutrophil elastase genes in immature myeloid cells. Mol Cell Biol 14: 5558–5568.
Okuda, T., Deursen, J. V., Hiebert, S. W., Grosveld, G. & Downing, J. R. (1996). AML1, the target of multiple chromosomal translocation in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321–330.
Okuda, T., Cai, Z., Yang, S., Lenny, N., Lyu, C. J., van Deursen, J. M., Harada, H. & Downing, J. R. (1998). Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood 91: 3134–3143.
Pace, U., Bockman, J. M., Miller, W. H. Jr, Dmitrovsky, E. & Goldberg, A. R. (1994). A ribozyme which discriminates in vitro between PML/RARα, the t(15;17)-associated fusion RNA of acute promyelocytic leukemia, and PML and RARα, the transcripts from the nonrearranged alleles. Cancer Res 54: 6365–6369.
Pachuk, C. J., Yoon, K., Moelling, K. & Coney, L. R. (1994). Selective cleavage of bcr-abl chimeric RNAs by a ribozyme targeted to non-contiguous sequences. Nucleic Acids Res 22: 301–307.
Rösl, F. (1992). A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res 20: 5243
Sakakura, C., Yamaguchi-Iwai, Y., Satake, M., Bae, S. C., Takahashi, A., Ogawa, E., Hagiwara, A., Takahashi, T., Murakami, A., Makino, K., Nakagawa, T., Kamada, N. & Ito, Y. (1994). Growth inhibition and induction of differentiation of t(8;21) acute myeloid leukemia cells by the DNA-binding domain of PEBP2 and the AML1/MTG8(ETO)-specific antisense oligonucleotide. Proc Natl Acad Sci USA 91: 11723–11727.
Schiffer, C. A., Lee, E. D., Tomiyasu, T., Wiernik, P. H. & Testa, J. R. (1989). Prognostic impact of cytogenetic abnormalities in patients with de novo acute nonlymphocytic leukemia. Blood 73: 263–270.
Shore, S. K., Nabissa, P. M. & Reddy, E. P. (1993). Ribozyme-mediated cleavage of the BCRABL oncogene transcript: in vitro cleavage of RNA and in vivo loss of P210 protein-kinase activity. Oncogene 8: 3183–3188.
Snyder, D. S., Wu, Y., Wang, J. L., Rossi, J. J., Swiderski, P., Kaplan, B. E. & Forman, S. J. (1993). Ribozyme-mediated inhibition of bcr-abl gene expression in a Philadelphia chromosome-positive cell line. Blood 82: 600–605.
Takahashi, A., Satake, M., Yamaguchi-Iwai, Y., Bae, S. C., Lu, J., Maruyama, M., Zhang, Y. W., Oka, H., Arai, N., Arai, K. & Ito, Y. (1995). Positive and negative regulation of granulocyte-macrophage colony-stimulating factor promoter activity by AML1-related transcription factor, PEBP2. Blood 86: 607–616.
Tanaka, T., Tanaka, K., Ogawa, S., Kurokawa, M., Mitani, K., Nishida, J., Shibata, Y., Yazaki, Y. & Hirai, H. (1995). An acute myeloid leukemia gene, AML1, regulates hematopoietic myeloid cell differentiation and transcriptional activation antagonistically by two alternative spliced forms. EMBO 14: 341–350.
Tanaka, T., Kurokawa, M., Ueki, K., Tanaka, K., Imai, Y., Mitani, K., Okazaki, K., Sagata, N., Yazaki, Y., Shibata, Y., Kadowaki, T. & Hirai, H. (1996). The extracellular signal-regulated kinase pathway phosphorylates AML1, an acute myeloid leukemia gene product, and potentially regulates its transactivation ability. Mol Cell Biol 16: 3967–3979.
Tashiro, S., Kyo, T., Tanaka, K., Oguma, N., Hashimoto, T., Dohy, H. & Kamada, N. (1992). The prognostic value of cytogenetic analysis in patients with acute nonlymphocytic leukemia treated with the same intensive chemotherapy. Cancer 70: 2809–2815.
Wang, Q., Stacy, T., Binder, M., Marin-Padilla, M., Sharpe, A. H. & Speck, N. A. (1996). Disruption of the cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA 93: 3444–3449.
Yergeau, D. A., Hetherington, C. J., Wang, Q., Zhang, P., Sharpe, A. H., Binder, M., Martin-Padilla, M., Tener, D. G. & Speck, N. A. (1997). Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML-ETO fusion gene. Nat Genet 15: 303–306.
Zhang, D., Fujioka, K., Hetherington, C. J., Shapiro, L. H., Chen, H., Look, T. & Tenen, D. G. (1994). Identification of a region which directs the monocytic activity of the colony-stimulating factor 1 (macrophage colony-stimulating factor) receptor promoter and binds PEBP2/CBF (AML1). Mol Cell Biol 14: 8085–8089.
Zhang, D., Hetherington, C. J., Meyers, S., Rhoades, K. L., Larson, C. J., Chen, H., Hiebert, S. W. & Tenen, D. G. (1996). CCAAT enhancer-binding protein (C/EBP) and AML1 (CBFα2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol Cell Biol 16: 1231–1240.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Matsushita, H., Kizaki, M., Kobayashi, H. et al. Induction of apoptosis in myeloid leukaemic cells by ribozymes targeted against AML1/MTG8. Br J Cancer 79, 1325–1331 (1999). https://doi.org/10.1038/sj.bjc.6690214
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bjc.6690214