Summary
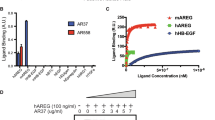
Colonic enterocytes, like many epithelial cells in vivo, are polarized with functionally distinct apical and basolateral membrane domains. The aims of this study were to characterize the endogenous epidermal growth factor (EGF)-like ligands expressed in two polarizing colon cancer cell lines, HCA-7 Colony 29 (HCA-7) and Caco-2, and to examine the effects of cell polarity on EGF receptor-mediated mitogenesis. HCA-7 and Caco-2 cells were grown on plastic, or as a polarized monolayer on Transwell filters. Cell proliferation was measured by 3H-thymidine incorporation and EGF receptor (EGFR) binding was assessed by Scatchard analysis. EGFR ligand expression was determined by Northern blot analysis, reverse transcription polymerase chain reaction, metabolic labelling and confocal microscopy. We found that amphiregulin (AR) was the most abundant EGFR ligand expressed in HCA-7 and Caco-2 cells. AR was localized to the basolateral surface and detected in basolateral-conditioned medium. Basolateral administration of neutralizing AR antibodies significantly reduced basal DNA replication. A single class of high-affinity EGFRs was detected in the basolateral compartment, whereas the apical compartment of polarized cells, and cells cultured on plastic, displayed two classes of receptor affinity. Basolateral administration of transforming growth factor alpha (TGF-α) or an EGFR neutralizing antibody also resulted in a dose-dependent stimulation or attenuation, respectively, of DNA replication. However, no mitogenic response was observed when these agents were added to the apical compartment or to confluent cells cultured on plastic. We conclude that amphiregulin acts as an autocrine growth factor in HCA-7 and Caco-2 cells, and EGFR ligand-induced proliferation is influenced by cellular polarity.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amemiya, K., Kurachi, H., Adachi, H., Morishige, K. I., Adachi, K., Imai, T. & Miyake, A. (1994). Involvement of epidermal growth factor (EGF)/EGF receptor autocrine and paracrine mechanism in human trophoblast cells: functional differentiation in vitro. J Endocrinol, 143: 291–301.
Auricchio, A., Di Domenico, M., Castoria, G., Bilancio, A. & Migliaccio, A. (1994). Epidermal growth factor induces protein tyrosine phosphorylation and association of p190 with ras-GTP-ase activating protein in Caco-2 cells. FEBS Lett 353: 16–20.
Basson, M. D., Beidler, D. R., Turowski, G., Zarif, A., Modlin, I. M., Jena, B. P. & Madri, J. A. (1994). Effect of tyrosine kinase inhibition on basal and epidermal growth factor-stimulated human Caco-2 enterocyte sheet migration and proliferation. J Cell Physiol 160: 491–501.
Basson, M. D., Modlin, I. M. & Madri, J. A. (1992). Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest 90: 15–23.
Bellot, F., Moolenaar, W., Kris, R., Mirakhur, B., Verlaan, I., Ullrich, A., Schlessinger, J. & Felder, S. (1990). High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol 110: 491–502.
Bishop, W. P. & Wen, J. T. (1994). Regulation of Caco-2 cell proliferation by basolateral membrane epidermal growth factor receptors. Am J Physiol 267: G892–G900.
Brown, C. L., Meise, K. S., Plowman, G. D., Coffey, R. J. & Dempsey, P. J. (1998). Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem 273: 17258–17268.
Chen, P., Gupta, K. & Wells, A. (1994). Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol 124: 547–555.
Chinery, R. & Cox, J. (1995). Modulation of epidermal growth factor effects on epithelial ion transport by intestinal trefoil factor. Br J Pharmacol 115: 77–80.
Coffey, R. J., Hawkey, C., Damstrup, L., Graves-Deal, R., Daniel, V. C., Dempsey, P. J., Chinery, R., Kirkland, S., DuBois, R. N., Jetton, T. L. & Morrow, J. D. (1997). Vectorial release and TGFα mediated production of prostaglanding is polarized colon carcinoma cell lines. Proc Natl Acad Sci USA 94: 657–662.
Cook, P. W., Pittelkow, M. R., Keeble, W. W., Graves-Deal, R., Coffey, R. J. Jr & Shipley, G. D. (1992). Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res 52: 3224–3227.
Culouscou, J-M, Remacle-Bonnet, M., Carlton, G. W., Plowman, G. D. & Shoyab, M. (1992). Colorectum cell-derived growth factor (CRDGF) is homologous to amphiregulin, a member of the epidermal growth factor family. Growth Factors 7: 195–205.
Danielson, P. E., Forss-Petter, S., Brow, M. A., Calavetta, L., Douglass, J., Milner, R. J. & Sutcliffe, J. G. (1988). p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA 7: 261–267
Defize, L. H., Boonstra, J., Meisenhelder, J., Kruijer, W., Tertoolen, L. G., Tilly, B. C., Hunter, T., Van Bergen En Henegouwen, P. M., Moolenaar, W. H. & De Laat, S. W. (1989). Signal transduction by epidermal growth factor occurs through the subclass of high affinity receptors. J Cell Biol 109: 2495–2507.
Dempsey, P. J. & Coffey, R. J. Jr (1994). Basolateral targeting and efficient consumption of transforming growth factor-α when expressed in Madin-Darby canine kidney cells. J Biol Chem 269: 16878–16889.
Dempsey, P. J., Meise, K., Yoshitake, Y., Nishikawa, K. & Coffey, R. J. (1997). Apical enrichment of human EGF presursor in Madin-Darby canine kidney cells involves preferential basolateral ectodomain cleavage sensitive to a metalloprotease inhibitor. J Cell Biol 138: 1–12.
Dobner, P. R., Kawasaki, E. S., Yu, L. Y. & Bancroft, F. C. (1981). Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci USA 78: 2230–2234.
Feldman, H. A. (1972). Mathematical theory of complex ligand-binding systems at equilibrium: some methods for parameter fitting. Analyt Biochem 48: 317–338.
Fowler, K. J., Walker, F., Alexander, W., Hibbs, M. L., Nice, E. C., Bohmer, R. M., Mann, G. B., Thumwood, C., Maglitto, R., Danks, J. A., Chetty, R., Burgess, A. W. & Dunn, A. R. (1995). A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA 92: 1465–1469.
Fuller, S. D. & Simons, K. (1986). Transferrin receptor polarity and recycling accuracy in “tight” and “leaky” strains of Madin-Darby canine kidney cells. J Cell Biol 103: 1767–1779.
Gex-Fabry, M. & Delisi, C. (1986). Regulation of interacting populations during endocytosis: models of growth factor-tumor promoter dynamics. Am J Physiol 250: R1123–R1132.
Halter, S. A., Dempsey, P. J., Matsui, Y., Stokes, M. K., Graves-Deal, R., Hogan, B. L. M. & Coffey, R. J. Jr (1992). Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-α. Characterization of mammary gland and skin proliferation. Am J Pathol 140: 1131–1146.
Hobert, M. & Carlin, C. (1995). Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J Cell Physiol 162: 434–446.
Johnson, G. R., Saeki, T., Gordon, A. W., Shoyab, M., Salomon, D. S. & Stromberg, K. (1992). Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol 118: 741–751.
Kirkland, S. C. (1985). Dome formation by a Human Colonic Adenocarcinoma cell line (HCA-7). Cancer Res 45: 3790–3795.
Lebivic, G., Quaroni, A. & Rodriguez-Boulan, E. (1991). Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol 113: 275–288.
Li, S., Plowman, G. D., Buckley, S. D. & Shipley, G. D. (1992). Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J Cell Physiol 153: 103–111.
Luetteke, N. C., Phillips, H. K., Qiu, T. H., Copeland, N. G., Earp, H. S., Jenkins, N. A. & Lee, D. C. (1994). The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 8: 399–413.
Maratos-Flier, E., Kao, C-YY, Verdin, E. M. & King, G. L. (1987). Reseptor-mediated vectorial transcytosis of epidermal growth factor by Madin-Darby canine kidney cells. J Cell Biol 105: 1595–1601.
Martinez-Palomo, A., Meza, I., Beaty, G. & Cereijido, M. (1980). Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol 87: 736–745.
Mayo, K. H., Nunez, M., Burke, C., Starbuck, C., Lauffenburger, D. & Savage, C. R. Jr (1989). Epidermal growth factor receptor binding is not a simple one-step process. J Biol Chem 264: 17838–17844.
Melton, D. A., Krieg, P. A., Rebagliati, M. R., Maniatis, T., Zinn, K. & Green, M. R. (1984). Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12: 7035–7056.
Milovic, V., Deubner, C., Zeuzem, S., Piiper, A., Caspary, W. F. & Stein, J. (1995). EGF stimulates polyamine uptake in Caco-2 cells. Biochem Biophys Res Commun 206: 962–968.
Pesonen, M. & Simons, K. (1983). Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. II. Immunological quantitation. J Cell Biol 97: 638–643.
Qi, C-F, Liscia, D. S., Normanno, N., Merlo, G., Johnson, G. R., Gullick, W. J., Ciardiello, F., Saeki, T., Brandt, R., Kim, N., Kenney, N. & Solomon, D. S. (1994). Expression of transforming growth factor alpha, amphiregulin and cripto-1 in human breast carcinomas. Br J Cancer 69: 903–910.
Scatchard, G. (1949). The attractions of proteins for small molecules and ions. Ann NY Acad Sci 51: 660–672.
Traverse, S., Seedorf, K., Paterson, H., Marshall, C. J., Cohen, P. & Ullrich, A. (1994). EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol 4: 694–701.
Wofsy, C., Goldstein, B., Lund, K. & Wiley, H. S. (1992). Implications of epidermal growth factor (EGF) induced EGF receptor aggregation. Biophys J 63: 98–110.
Ziober, B. L., Willson, J. K., Hymphrey, L. E., Childress-Fields, K. & Brattain, M. G. (1993). Autocrine transforming growth factor-alpha is associated with progression of transformed properties in human colon cancer cells. J Biol Chem 268: 691–698.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Damstrup, L., Kuwada, S., Dempsey, P. et al. Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. Br J Cancer 80, 1012–1019 (1999). https://doi.org/10.1038/sj.bjc.6690456
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bjc.6690456
Keywords
This article is cited by
-
Loss of miR-24-3p promotes epithelial cell apoptosis and impairs the recovery from intestinal inflammation
Cell Death & Disease (2021)
-
Effect of blockade of the EGF system on wound healing in patients vaccinated with CIMAvax® EGF
World Journal of Surgical Oncology (2013)
-
Identification of the angiogenic gene signature induced by EGF and hypoxia in colorectal cancer
BMC Cancer (2013)
-
Proneoplastic effects of PGE2mediated by EP4 receptor in colorectal cancer
BMC Cancer (2009)
-
Bile Acid Alone, or in Combination with Acid, Induces CDX2 Expression Through Activation of the Epidermal Growth Factor Receptor (EGFR)
Journal of Gastrointestinal Surgery (2009)