Summary
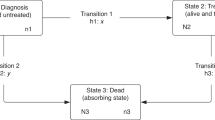
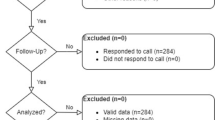
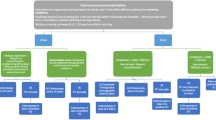
Colorectal cancers emerging after a negative Haemoccult II® are described in the context of a first round of mass screening in the Department of Calvados (France), from April 1991 to the end of December 1994. People with a cancer occurring after a negative test until 31 December 1995 were identified by a local cancer registry. Incidence was calculated and the programme sensitivity was estimated. The incidence of cancer emerging after a negative test was 57.7 per 100 000, i.e. half of the calculated incidence in the reference group (141.6 per 100 000). These cancers did not differ from those of either the non-responder or reference groups, in particular for the stage of extension. The programme sensitivity was globally higher than that estimated in European trials: 77.2, 66.3 and 55.9%, 1, 2 and 3 years after the test respectively. Programme sensitivity was higher for distal colon cancer 1 year after the test, which is probably due to the relatively slow growth of this subsite.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allison, JE, Feldman, R & Tekawa, IS (1990). Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity and predicting value. Ann Intern Med 112: 328–333.
Benhamiche, AM, Faivre, J, Menegoz, F & Grosclaude, P (1997). Les cancers digestifs en France à l’aube de l’an 2000. Hepato-Gastro 4: 8–12.
Church, TR, Ederer, F & Mandel, JS (1997). Faecal occult blood screening in the Minnesota study: sensitivity of the screening test. J Natl Cancer Inst 89: 1440–1448.
Dukes, CE (1932). The classification of cancer of the rectum. J Pathol Bacteriol 35: 323–332.
Faivre, J, Grosclaude, P, Launoy, G, Arveux, P, Raverdy, N, Menegoz, F, Schaffer, P, Daures, JP & De Vathaire, F (1997). Les cancers digestifs en France. Distribution géographique et estimation de l’incidence nationale. Gastroentérol Clin Biol 21: 174–180.
Hardcastle, JD, Chamberlain, JO, Robinson, MH, Moss, SM, Amar, SS, Balfour, TW, James, PD & Mangham, CM (1996). Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 348: 1472–1477.
Kronborg, O, Fenger, C, Sonderaard, O, Pedersen, KM & Olsen, J (1987). Initial mass screening for colorectal cancer with faecal occult blood test. Scand J Gastroenterol 22: 677–686.
Kronborg, O, Fenger, C, Olsen, J, Bech, K & Sonderaard, O (1989). Repeated screening for colorectal cancer with faecal occult blood test. A prospective randomised study at Funen, Denmark. Scand J Gastroenterol 24: 599–606.
Kronborg, O, Fenger, C, Olsen, J, Jorgensen, OD & Sondergaard, O (1996). Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 348: 1467–1471.
Launoy, G, Herbert, C, Reaud, JM, Thezee, Y, Tichet, J, Maurel, J, Ollivier, V, Pegulu, L, Caces, E, Valla, A & Gignoux, M (1995). Haemoccult test properties according to type and number of positive slides in mass screening for colorectal cancer. Br J Cancer 72: 1043–1046.
Launoy, G, Herbert, C, Vallee, JP, Desoubeaux, N, Reaud, JM, Ollivier, V, Bouvier, V, Flachs, A, Arsene, D, Valla, A & Gignoux, M (1996). Le dépistage de masse du cancer colorectal en France. Expérience auprès de 165.000 personnes dans le Calvados. Gastroentérol Clin Biol 20: 228–236.
Launoy, G, Smith, TC, Duffy, WS & Bouvier, V (1997). Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer 73: 220–224.
Mandel, JS, Bond, JH, Church, TR, Snover, DC, Bradely, MB, Schuman, LM & Ederer, F (1993). Reducing mortality from colorectal cancer by screening for faecal occult blood. N Engl J Med 328: 1365–1371.
Robinson, MH, Moss, SM, Hardcastle, JD, Whynes, DK, Chamberlain, J & Mangham, CM (1995). Effect of retesting with dietary restriction in Haemoccult screening for colorectal cancer. J Med Screen 2: 41–44.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Bouvier, V., Launoy, G., Herbert, C. et al. Colorectal cancer after a negative Haemoccult II® test and programme sensitivity after a first round of screening: The experience of the Department of Calvados (France). Br J Cancer 81, 305–309 (1999). https://doi.org/10.1038/sj.bjc.6990692
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bjc.6990692