Abstract
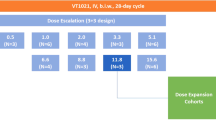
Based on the already known in vitro synergy between paclitaxel (taxol), cisplatin and oxazophosphorine cytostatics and the broad spectrum of activity of the above drugs we sought to evaluate the paclitaxel (taxol)-ifosfamide-cisplatin (PIC) combination in the outpatient setting in individuals with a variety of advanced solid tumours. Cohorts of patients were entered into six successive dose levels (DLs) with drug doses ranging as follows: paclitaxel 135–215 mg m–2 day 1 – (1 h infusion), ifosfamide 4.5–6.0 g m–2 (total dose) – divided over days 1 and 2, and cisplatin 80–100 mg m–2 (total) – divided over days 1 and 2. Granulocyte colony-stimulating factor was given from day 5 to 14. Forty-two patients were entered. Eighteen patients had 2–8 cycles of prior chemotherapy with no taxanes or ifosfamide (cisplatin was allowed). The regimen was tolerated with outpatient administration in 36/42 patients. Toxicities included: grade 4 neutropenia for ≤ 5 days in 27% of cycles; 5 episodes of febrile neutropenia in three patients at DL-III, -V and -VI. Grade 3/4 thrombocytopenia and cumulative grade 3 anaemia were seen in 7% and 13% of cycles respectively. Three cases of severe grade 3 neuromotor/sensory neuropathy were recorded at DL-II, -III, and -V, all after cycle 3. The maximum tolerated dose was not formally reached at DL-V, but because of progressive anaemia and asthenia/fatigue, it was decided to test a new DL-VI with doses of paclitaxel 200 mg m–2, ifosfamide 5.0 g m–2 and cisplatin 100 mg m–2; this appeared to be tolerable and is recommended for further phase II testing. The response rate was 47.5% (complete response + partial response: 20/42). The PIC regimen appears to be feasible and safe in the outpatient setting. Care should be paid to neurotoxicity. Phase II studies are starting in non-small-cell lung cancer, ovarian cancer and head and neck cancer at DL-VI. © 2000 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bajorin DF, McCaffrey JA, Hilton S, Mazumdar M, Kelly WK, Scher HI, Spicer J, Herr H and Higgins G (1998) Treatment of patients with transitional-cell carcinoma of the urothelial tract with ifosfamide, paclitaxel, and cisplatin: a phase II trial. J Clin Oncol 16: 2722–2727
Bunnell CA, Thompson L, Buswell L, Berkowitz R, Muto M, Sheets E and Shulman LN (1998) A phase I trial of ifosfamide and paclitaxel with granulocyte-colony stimulating factor in the treatment of patients with refractory solid tumors. Cancer 82: 561–566
Donnellan PP and Crown JP (1997) The development of docetaxel (taxotere) in non-small cell lung cancer. Docetaxel in new combinations and new schedules: an overview of ongoing and future developments. Semin Oncol 24: 18–21
Greco FA and Hainsworth JD (1995) One hour paclitaxel infusion schedule: a phase I/II comparative trial. Semin Oncol 22: 118–123
Horwich A, Dearnaley DP, Norman A, Nicolls J and Hendry WF (1994) Accelerated chemotherapy for poor prognosis germ cell tumours. Eur J Cancer 30A: 1607–1611
Huizing MT, van Warmerdarm JC, Rosing H, Schaefers MCW, Lai A, Helmerhorst TJM, Veenhof CHN, Birkhofer MJ, Rodenhuis S, Beijnen JH and ten Bokkel Huinik WW (1997) Phase I and pharmacologic study of the combination paclitaxel and carboplatin as first-line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol 15: 1953–1964
Kearns CM, Gianni L and Egorin MJ (1995) Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 22: 16–23
Kennedy MJ, Armstrong D, Donehower R, Noe D, Sartorius S, Chen T-L, Bowling K and Rowinsky E (1994) The hematologic toxicity of the Taxol/Cytoxan doublet is sequence-dependent. Proc Am Soc Clin Oncol 13: 74 (abstract 342)
Klaassen U, Harstrick A, Schleucher N, Vanhoefer U, Schroder J, Wilke H and Seeber S (1996) Activity and schedule-dependent interactions of paclitaxel, etoposide and hydroperoxy-ifosfamide in cisplatin-sensitive and -refractory human ovarian carcinoma cell lines. Br J Cancer 74: 224–228
Liebmann JE, Fisher J and Teague D (1994) Sequence dependence of paclitaxel (Taxol) combined with cisplatin or alkylators in human cancer cells. Oncol Res 6: 25–31
Lind MJ, McGowan AT, Hadfield JA, Thatcher N, Crowther D and Fox BW (1989) The effect of ifosfamide and its metabolites on intracellular glutathione levels in vitro and in vivo. Biochem Pharmacol 38: 1835–1840
Motzer RJ, Bajorin DF, Bosl GJ, Reich L, Tong W and Green GA (1997) Paclitaxel containing first-line salvage therapy selected by risk for patients with germ cell tumors. Proc Am Soc Clin Oncol 13: 322 (abstract 1146)
Pagani O, Sessa C, Martinelli G, Cerny T, de Jong J, Goldhirsh A, Zimatore M and Cavalli F (1997) Dose-finding study of paclitaxel and cyclophosphamide in advanced breast cancer. Ann Oncol 8: 655–661
Palackdharry CS (1997) Phase I trial of dose-escalated paclitaxel and carboplatin in combination with ifosfamide and filgrastim: preliminary results. Semin Oncol 24: 108–112
Parker RJ, Dabholkar MD, Lee KB, Bostick-Bruton F and Reed E (1993) Taxol effect on cisplatin sensitivity and cisplatin cellular accumulation in human ovarian cancer cells. J Natl Cancer Inst Monogr 15: 83–88
Pronk LC, Schrijvers D, Schellens JHM, de Bruijn EA, Planting ASTh, Locci-Tonelli D, Grouit V, Verweij J and van Oosterom AT (1998) Phase I study of docetaxel and ifosfamide in patients with advanced solid tumors. Br J Cancer 77: 153–158
Reed E, Kohn EC, Sarosy G, Dabholkar M, Davis P, Jacob J and Maher M (1995) Paclitaxel, cisplatin, and cyclophosphamide in human ovarian cancer: molecular rationale and early clinical results. Semin Oncol 22: 90–96
Rowinsky EK, Chaudhry V, Forastiere AA, Sartorius SE, Ettinger DS, Grochow LB, Lubejko BG, Cornblath DR and Donehower RC (1993) Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J Clin Oncol 11: 2010–2020
Shin DM, Glisson BS, Khuri FR, Ginsberg L, Papadimitrakopoulou V, Lee JJ, Lawhorn K, Gillenwater AM, Ang K-K, Clayman GL, Callender DL, Hong WK and Lippman SM (1998) Phase II trial of paclitaxel, ifosfamide, and cisplatin in patients with recurrent head and neck squamous cell carcinoma. J Clin Oncol 16: 1325–1330
Tishler RB, Geard CR, Hall EJ and Schiff PB (1992 a) Taxol sensitizes human astrocytoma cells to radiation. Cancer Res 52: 3495–3497
Tishler RB, Schiff PB, Geard CR and Hall EJ (1992 b) Taxol: a novel radiation sensitizer. Int J Rad Oncol Biol Phys 22: 613–617
Tolcher AW (1996) Paclitaxel couplets with cyclophosphamide or cisplatin in metastatic breast cancer. Semin Oncol 23: 37–43
Tsavaris N and Kosmas C (1998) Risk of severe acute hypersensitivity reactions after rapid (less than 1-hour) paclitaxel infusion. Cancer Chemother Pharmacol 42: 509–511
Tsavaris N, Polyzos A, Kosmas C, Giannikos L and Gogas J (1997) A feasibility study of one-hour paclitaxel infusion in solid tumors. Cancer Chemother Pharmacol 40: 353–357
Tsavaris N, Fountzilas G, Mylonakis N, Athanassiadis A, Kosmas C, Karakousis C, Bacoyiannis CH and Kosmidis P (1998) A randomized comparative study of antiemetic prophylaxis with ondansetron in a single 32 mg loading dose vs 8 mg every six hours, in patients under cisplatin-based chemotherapy. Oncology 55: 513–516
Zanetta G, Lissoni A, Pellegrino A, Sessa C, Colombo N, Gueli-Alletti D and Mangioni C (1998) Neoadjuvant chemotherapy with cisplatin, ifosfamide and paclitaxel for locally advanced squamous-cell cervical cancer. Ann Oncol 9: 977–980
Zaniboni A, Meriggi F, Rizzi A, Alghisi A, Pascarella A, Bozzola G, Mutti S and Marini G (1997) Paclitaxel, ifosfamide, and carboplatin for the treatment of stages IIIB and IV non-small cell lung cancer. Semin Oncol 24: 70–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kosmas, C., Tsavaris, N., Polyzos, A. et al. Phase I study of dose-escalated paclitaxel, ifosfamide, and cisplatin (PIC) combination chemotherapy in advanced solid tumours. Br J Cancer 82, 300–307 (2000). https://doi.org/10.1054/bjoc.1999.0919
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.1999.0919
Keywords
This article is cited by
-
Evaluation of the paclitaxel–ifosfamide–cisplatin (TIP) combination in relapsed and/or metastatic cervical cancer
British Journal of Cancer (2009)
-
Docetaxel–ifosfamide combination in patients with HER2-non-overexpressing advanced breast cancer failing prior anthracyclines
Investigational New Drugs (2007)
-
Phase I–II study of docetaxel and ifosfamide combination in patients with anthracycline pretreated advanced breast cancer
British Journal of Cancer (2003)