Abstract
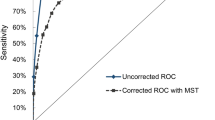
We used a nested case–control design on data from men in four prospective studies (from the UK, Maryland in the USA, and two from Finland) with available stored serum samples to determine whether there was an advantage in measuring both free prostate-specific antigen (PSA) and total PSA as a potential screening test for prostate cancer. Of these men, 247 were verified through national vital statistics offices as having died of prostate cancer, or having developed the disease, and 953 men who did not develop prostate cancer (controls) were selected, matched to cases for age, study centre and sample storage duration. Fixing the false-positive rate at 1%, the prostate cancer detection rate (sensitivity) over the 3 years following serum collection (based on 14 cancers) increased from an estimated 95% using total PSA to 97% using free and bound PSA (that is, bound to α-antichymotrypsin which together with the free form is total PSA). Over a 6-year period (based on 41 cancers) a similar difference occurred (52% and 56% detection rates respectively). We conclude that there is no material advantage in adding free to total PSA in prostate cancer screening trials. © 2000 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arcangeli CG, Ornstein DK, Keetch DW and Andriole GL (1997) Prostate-specific antigen as a screening test for prostate cancer. Urol Clin North Am 24: 299–306
Auvinen A, Rietbergen JBW, Denis LJ, Schröder FH and Prorok PC for the International Prostate Cancer Screening Trial Evaluation Group (1996) Prospective evaluation plan for randomised trials of prostate cancer screening. J Med Screening 3: 97–104
Ballentine Carter H, Pearson JD, Metter J, Brant LJ, Chan DW, Andres R, Fozard JL and Walsh PC (1992) Longitudinal evaluation of prostate specific antigen levels in men with and without prostate disease. JAMA 267: 2215–2220
Gann PH, Hennekens CH and Stampfer MJ (1995) A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 273: 289–294
Helzlsouer KJ, Newby J and Comstock GW (1992) Prostate-specific antigen levels and subsequent prostate cancer: potential for screening. Cancer Epidemiol Biomarkers 1: 537–540
Jacobsen SJ, Bergstralh EJ, Guess HA, Katusic SK, Klee GG, Oesterling JE and Lieber MM (1996) Predictive properties of serum prostate specific antigen testing in a community-based setting. Arch Int Med 156: 2462–2468
Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Teppo L and Hakama M (1988) Serum vitamin E, serum selenium and the risk of gastrointestinal cancer. Int J Cancer 42: 846–850
Lein M, Koenig F, Jung K, McGovern FJ, Skates SJ, Schnorr D and Loening SA (1998) The percentage of free prostate specific antigen is an age-independent tumour marker for prostate cancer: establishment of reference ranges in a large population of healthy men. Br J Urol 82: 231–236
Orstein DK, Snith DS, Rao GS, Basler JW, Ratliff TL and Catalona WJ (1997) Biological variation of total, free and percent free serum prostate specific antigen levels in screening volunteers. J Urol 157: 2179–2182
Parkes C, Wald NJ, Murphy P, George L, Watt HC, Kirby R, Knekt P, Helzlsouer KJ and Tuomilehto J (1995) Prospective observational study to assess value of prostate specific antigen as screening test for prostate cancer. Br Med J 311: 1340–1343
Piironen T, Pettersson K, Suonpaa M, Stenman UH, Oesterling JE, Lovgren T and Lilja H (1996) In vitro stability of free prostate specific antigen (PSA) and prostate specific antigen (PSA) complexed to α1-antichymotrypsin in blood samples. Urology 48: 81–87
Puska P, Tuomilehto J, Salonen JT, Neittaanmaki L, Maki J, Virtamo J, Nissinen A, Koskela K and Takalo T (1979) Changes in coronary risk factors during comprehensive 5-year community programme to control cardiovascular diseases (the North Karelia Project). Br Med J ii: 1173–1178
Royston P and Thompson SG (1992) Model-based screening by risk with application to Down's syndrome. Stat Med 11: 257–268
Stenman UH, Hakama M, Knekt P, Aromaa A, Teppo L and Leinonen J (1994) Serum concentrations of prostate specific antigen and its complex with α1-antichymotrypsin before diagnosis of prostate cancer. Lancet 344: 1594–1598
Stenman UH, Leinonen J, Alfthan H, Ranniko S, Tuhkanen K and Alfthan O (1991) A complex between prostate specific antigen and α1-antichymotrypsin is the major form of prostate specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res 51: 222–226
Wald NJ, Thompson SG, Densem JW, Boreham J and Bailey A (1987) Serum vitamin E and subsequent risk of cancer. Br J Cancer 56: 69–72
Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, Haddow JE, Knight GJ, Palomaki GE and Canick JA (1998) Maternal serum screening for Down's syndrome in early pregnancy. Br Med J 297: 883–887
Whittemore AS, Lele C, Friedman GD, Stamey T, Vogelman JH and Orentreich N (1995) Prostate-specific antigen as predictor of prostate cancer in black men and white men. J Natl Cancer Inst 87: 354–360
Woodrum D, French C and Shamel B (1996) Stability of free prostate specific antigen in serum samples under a variety of sample collection and sample storage conditions. Urology 48: 33–39
Woodrum DL, Brawer MK, Partin AW, Catalona WJ and Southwick PC (1998) Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol 159: 5–12
Wu JT and Stephenson RA (1997) Microplate assay for free PSA, PSA ACT and total PSA: assay characteristics, selection of calibrators and in vitro stability studies. In: First Consultation on Prostate Cancer, Murphy G, Griffiths K, Denis L, Khoury S, Chatelain C, Crockett AT (eds). Scientific Communication International: London
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wald, N., Watt, H., George, L. et al. Adding free to total prostate-specific antigen levels in trials of prostate cancer screening. Br J Cancer 82, 731–736 (2000). https://doi.org/10.1054/bjoc.1999.0988
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.1999.0988