Abstract
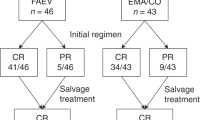
Persistent gestational trophoblastic disease is potentially fatal, but the majority of patients are cured with chemotherapy. Any developments in treatment are therefore being directed towards maintaining efficacy and reducing toxicity. We evaluated efficacy and toxicity of methotrexate, etoposide and dactinomycin (MEA) as first-line therapy for high risk disease and etoposide and dactinomycin (EA) as second-line therapy for methotrexate-refractory low risk disease in a retrospective analysis of 73 patients (38 MEA, 35 EA) treated since 1986 at a supra-regional centre. The median follow-up period was 5.5 years and the median number of cycles received was 7. The overall complete response rate was 85% (97% for EA, 75% for MEA). Of eight patients who failed to respond, four have since died and four were cured with platinum-based chemotherapy. Alopecia was universal. Grade II or worse nausea, emesis, or stomatitis was observed in 29%, 30% and 37% respectively. Fifty-one per cent experienced grade II/III anaemia, 8% grade II or higher thrombocytopenia and 64% grade III or IV neutropenia; in six cases this was complicated by sepsis. Fifty-four per cent of patients went on to have a normal pregnancy. No patient has developed a second malignancy. In conclusion, the MEA and EA chemotherapy regimens for persistent trophoblastic disease are very well tolerated, do not appear to affect future fertility and are associated with excellent, sustained complete response rates. © 2000 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ayhan A, Yapar EG and Deren O et al (1992) Remission rates and significance of prognostic factors in gestational trophoblastic tumours. J Reprod Med 37: 461–465
Bagshawe KD (1976) Risk and prognostic factors in trophoblastic neoplasia. Cancer 38: 1373–1385
Bagshawe KD, Dent J and Newlands ES et al (1989) The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumours. Br J Obstet Gynaecol 96: 795–802
Berkowitz RS and Goldstein DP (1997) Presentation and management of persistent gestational trophoblastic disease and gestational trophoblastic tumours in the USA. In: Gestational Trophoblastic Disease, 1st edn, Hancock BW, Newlands ES, Berkowitz RS (eds), pp 159–172, Chapman and Hall: London
Bower M, Newlands ES and Holden L et al (1997) EMA/CO for high-risk gestational trophoblastic tumours: results from a cohort of 272 patients. J Clin Oncol 15: 2636–2643
Doreen MS (1993) Gestational trophoblastic diseases: a review of their presentation and management. Clin Oncol 5: 46–56
DuBreschter B, Berkowitz RS and Goldstein DP et al (1987) Metastatic gestational trophoblastic disease: experience at the New England Trophoblastic Disease Centre, 1965–1985. Obstet Gynaecol 69: 390–395
Dubuc-Lissor J, Sweizig S and Schlaerth JB et al (1989) Metastatic gestational trophoblastic disease: a comparison of prognostic classification systems. Gynaecol Oncol 45: 40–45
FIGO Oncology Committee Report (1992) Int J Gynaecol 39: 149–150
Gillespie AM, Siddiqui N, Coleman RE and Hancock BW (1999) Gestational trophoblastic disease: does central nervous system chemoprophylaxis have a role? Br J Cancer 79: 1270–1272
Goldstein DP and Berkowitz RS (1995) Prophylactic chemotherapy of complete molar pregnancy. Semin Oncol 22: 157–160
Hammond CB, Borchert LG and Tyrey L et al (1973) Treatment of metastatic disease: good and poor prognosis. Am J Obstet Gynaecol 115: 451–457
Hancock BW (1997) Difference in management and treatment: a critical appraisal. In: Gestational Trophoblastic Disease, 1st edn, Hancock BW, Newlands ES, Berkowitz RS (eds), pp 213–216, Chapman and Hall: London
Kennedy AW (1995) Persistent non-metastatic gestational trophoblastic disease. Semin Oncol 22: 161–165
Lurain JR (1994) High-risk metastatic gestational trophoblastic tumours: current management. J Reprod Med 39: 217–222
Newlands ES (1997) Presentation and management of persistent gestational trophoblastic disease and gestational trophoblastic tumours in the UK. In: Gestational Trophoblastic Disease, 1st edn., Hancock BW, Newlands ES, Berkowitz RS (eds), pp 143–156, Chapman and Hall: London
Newlands ES, Bagshawe KD and Begent RHJ et al (1986) Developments in chemotherapy for medium and high risk patients with gestational trophoblastic tumours, 1979–84. Br J Obstet Gynaecol 93: 63–69
Newlands ES, Bagshawe KD and Begent RHJ et al (1991) Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen in high risk gestational trophoblastic tumours, 1979–1989. Br J Obstet Gynaecol 98: 550–557
Quinn M, Murray J and Friedlander M et al (1994) EMACO in high-risk gestational trophoblastic disease; the Australian experience. Aust NZ J Obstet Gynaecol 34: 90–92
Sheridan E, Hancock BW and Smith SC et al (1993) Gestational trophoblastic disease: experience of the Sheffield (United Kingdom) supra regional screening and treatment service. Int J Oncol 3: 149–155
Soper JT, Clarke-Pearson DL and Berchuck A et al (1994a) 5-day methotrexate for women with metastatic gestational trophoblastic disease. Gynaecol Oncol 54: 76–79
Soper JT, Evans AC and Clarke-Pearson DL et al (1994b) Alternating weekly chemotherapy with etoposide-methotrexate-dactinomycin/cyclophosphamide-vincristine for high risk gestational trophoblastic disease. Obstet Gynaecol 83: 113–117
Welsh E, Gillespie AM and Hancock BW (1999) Assessing and staging trophoblastic tumours – a comparison of the various systems. Br J Cancer 80: 110
WHO World Health Organization Geneva (1987) Trophoblastic diseases. WHO Tech Rep Ser 692
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Dobson, L., Lorigan, P., Coleman, R. et al. Persistent gestational trophoblastic disease: results of MEA (methotrexate, etoposide and dactinomycin) as first-line chemotherapy in high risk disease and EA (etoposide and dactinomycin) as second-line therapy for low risk disease. Br J Cancer 82, 1547–1552 (2000). https://doi.org/10.1054/bjoc.2000.1176
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2000.1176
Keywords
This article is cited by
-
The efficacy and toxicity of 4-day chemotherapy with methotrexate, etoposide and actinomycin D in patients with choriocarcinoma and high-risk gestational trophoblastic neoplasia
International Journal of Clinical Oncology (2020)
-
External validation of serum hCG cutoff levels for prediction of resistance to single-agent chemotherapy in patients with persistent trophoblastic disease
British Journal of Cancer (2009)
-
A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia
British Journal of Cancer (2007)
-
Gestational trophoblastic neoplasia: The management of relapsing patients and other recent advances
Current Oncology Reports (2004)
-
Low-risk persistent gestational trophoblastic disease treated with low-dose methotrexate: efficacy, acute and long-term effects
British Journal of Cancer (2003)