Abstract
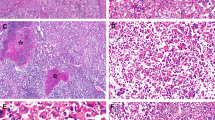
To investigate the relationship between the expression of p21WAF1/CIP1 protein and p53 status and the possible role of the two proteins in hepatocellular carcinomas (HCCs), we examined the expression of p21WAF1/CIP1 and p53 immunohistochemically in 81 tumours from 65 patients with hepatocellular carcinoma. p21WAF1/CIP1 protein was absent from 59 of 81 tumours (72.8%), and altered p53 expression was found in 43 (53.1%). p21WAF1/CIP1 expression was significantly associated with p53 status (P= 0.0008); 38 of 59 tumours lacking p21WAF1/CIP1 protein were accompanied by altered p53 expression. Further analyses showed that p21WAF1/CIP1 expression was inversely correlated with p53 expression in hepatitis C virus (HCV)-related HCCs, but not in HBV-related hepatocellular carcinomas and hepatocellular carcinomas without viral infection. All 11 tumours with intrahepatic metastasis showed altered p21WAF1/CIP1 or p53 expression. In contrast, no intrahepatic metastasis was found in any of the 17 tumours without abnormal expression of either of the two proteins. These results suggest that: (1) different modes of p21WAF1/CIP1 regulation are involved in HCCs differing in their hepatitis viral infection status, and p21WAF1/CIP1 expression appears to be predominantly related to altered p53 in HCV-related HCCs; (2) disruption of the p53–p21WAF1/CIP1 cell- cycle-regulating pathway may contribute to malignant progression of HCC. © 2000 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Cote RJ, Dunn MD, Chatterjee SJ, Stein JP, Shi SR, Tran QC, Hu SX, Xu HJ, Groshen S, Taylor CR, Skinner DG and Benedict WF (1998) Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res 58: 1090–1094
Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ and Reed SI (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825
El-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, Wilman KG, Mercer WE, Kastan MB, Kohn KW, Elledge SJ, Kinzler KW and Vogelstein B (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 54: 1169–1174
Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen S-C, Nichols PW, Skinner DG, Jones PA and Cote RJ (1994) Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 331: 1259–1264
Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R and Prives C (1992) Wild-type p53 activates transcription in vitro. Nature 358: 83–86
Finlay CA, Hinds PW, Tan T-H, Eliyahu D, Oren M and Levine AJ (1988) Activating mutations for transformation by p53 produce a gene product that forms an hsc70–p53 complex with an altered half-life. Mol Cell Biol 8: 531–539
Furutani M, Arii S, Tanaka H, Mise M, Niwano M, Harada T, Higashitsuji H, Imamura M and Fujita J (1997) Decreased expression and rare somatic mutation of the CIP1/WAF1 gene in human hepatocellular carcinoma. Cancer Lett 111: 191–197
Gu Y, Turck CW and Morgan DO (1993) Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 366: 707–710
Hall PA, Ray A, Lemoine NR, Midgley CA, Krausz T and Lane DP (1991) p53 immunostaining as a marker of malignant disease in diagnostic cytopathology. Lancet 338: 513
Harper JW, Adami GR, Wei N, Keyomarsi K and Elledge SJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805–816
Hui A-M and Makuuchi M (1999) Molecular basis of multistep hepatocarcinogenesis: genetic and epigenetic events. Scand. J Gastroenterol 34: 737–742
Hui A-M, Sakamoto M, Kanai Y, Ino Y, Gotoh M and Hirohasi S 1996a) Cyclin-dependent kinase inhibitors and hepatocarcinogenesis. In: Recent Advances in Gastroenterological Carcinogenesis, Tahara E Sugimachi K Oohara T (eds), pp. 481–485. Monduzzi Editore: Bologna
Hui A-M, Sakamoto M, Kanai Y, Ino Y, Gotoh M, Yokota J and Hirohashi S 1996b) Inactivation of p16INK4in hepatocellular carcinoma. Hepatology 24: 575–579
Hui A-M, Kanai Y, Sakamoto M, Tsuda H and Hirohashi S (1997) Reduced p21WAF1/CIP1expression and p53 mutation in hepatocellular carcinomas. Hepatology 25: 575–579
Hui A-M, Makuuchi M and Li X 1998a) Cell cycle regulators and human hepatocarcinogenesis. Hepato-Gastroenterology 45: 1635–1642
Hui A-M, Sun L, Kanai Y, Sakamoto M and Hirohashi S 1998b) Reduced p27kip1expression in hepatocellular carcinomas. Cancer Lett 132: 67–73
Hui A-M, Cui X, Makuuchi M, Li X, Shi Y-Z and Takayama T 1999a) Decreased p27Kip1expression and cyclin D1 overexpression, alone and in combination, influence recurrence and survival of patients with resectable extrahepatic bile duct carcinoma. Hepatology 30: 1167–1173
Hui A-M, Li X, Makuuchi M, Takayama T and Kubota K 1999b) Over-expression and lack of retinoblastoma protein are associated with tumor progression and metastasis in hepatocellular carcinoma. Int J Cancer 84: 604–608
Hui A-M, Shi Y-Z, Li X, Takayama T and Makuuchi M (2000) Loss of p16INK4protein, alone and together with loss of retinoblastoma protein, correlate with hepatocellular carcinoma progression. Cancer Lett,
Hunter T and Pines J (1994) Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell 79: 573–582
Iggo R, Gatter K, Bartek J, Lane D and Harris AL (1990) Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet 335: 675–679
Jiang H, Lin J, Su ZZ, Herlyn M, Kerbel RS, Weissman BE, Welch DR and Fisher PB (1995) The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene 10: 1855–1864
Li X, Hui A-M, Takayama T, Cui X, Shi Y-Z and Makuuchi M (2000) Altered p21WAF1/CIP1expression is associated with poor prognosis in extrahepatic bile duct carcinoma. Cancer Lett,
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM and Pagano M (1990) Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 3: 231–234
Maki CG and Howley PM (1997) Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol 17: 355–363
Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE and Givol D (1994) Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res 54: 3391–3395
Naka T, Toyota N, Kaneko T and Kaibara N (1998) Protein expression of p53, p21WAF1, and Rb as prognostic indicators in patients with surgically treated hepatocellular carcinoma. Anticancer Res 18: 555–564
Ng IO, Lai EC, Chan AS and So MK (1995) Overexpression of p53 in hepatocellular carcinomas: a clinicopathological and prognostic correlation. J Gastroenterol Hepatol 10: 250–255
Noda A, Ning Y, Venable SF, Pereira-Smith OM and Smith JR (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 211: 90–98
Ogawa M, Maeda K, Onoda N, Chung YS and Sowa M (1997) Loss of p21WAF1/CIP1expression correlates with disease progression in gastric carcinoma. Br J Cancer 75: 1617–1620
Okuda K (1997) Hepatitis C virus and hepatocellular carcinomas. In: Liver Cancer, Okuda K Tabor E (eds), pp. 39–50. Churchill Livingstone: Edinburgh
Qin LF, Ng IO, Fan ST and Ng M (1998) p21/WAF1, p53 and PCNA expression and p53 mutation status in hepatocellular carcinoma. Int J Cancer 79: 424–428
Ray RB, Steele R, Meyer K and Ray R (1998) Hepatitis C virus core protein represses p21WAF1/CIP1/Sid1promoter activity. Gene 208: 331–336
Sarnow P, Ho YS, Williams J and Levine AJ (1982) Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kDa cellular protein in transformed cells. Cell 28: 387–394
Sheikh MS, Rochefort H and Garcia M (1995) Overexpression of p21WAF1/CIP1induces growth arrest, giant cell formation and apoptosis in human breast carcinoma cell lines. Oncogene 11: 1899–1905
Somasundaram K and El-Deiry WS (1997) Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene 14: 1047–1057
Steegenga WT, van Laar T, Riteco N, Mandarino A, Shvarts A, van der Eb AJ and Jochemsen AG (1996) Adenovirus E1A proteins inhibit activation of transcription by p53. Mol Cell Biol 16: 2101–2109
Waga S, Hannon GJ, Beach D and Stillman B (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369: 574–578
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R and Beach D (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366: 701–704
Yew PR and Berk AJ (1992) Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 357: 82–85
Zhang W, Grasso L, McClain CD, Gambel AM, Cha Y, Travali S, Deisseroth AB and Mercer WE (1995) p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophag differentiation. Cancer Res 55: 668–674
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shi, YZ., Hui, AM., Takayama, T. et al. Reduced p21WAF1/CIP1 protein expression is predominantly related to altered p53 in hepatocellular carcinomas. Br J Cancer 83, 50–55 (2000). https://doi.org/10.1054/bjoc.2000.1310
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2000.1310
Keywords
This article is cited by
-
Human amniotic membrane conditioned medium inhibits proliferation and modulates related microRNAs expression in hepatocarcinoma cells
Scientific Reports (2019)
-
Effect of PAK1 gene silencing on proliferation and apoptosis in hepatocellular carcinoma cell lines MHCC97-H and HepG2 and cells in xenograft tumor
Gene Therapy (2018)
-
EGFR and SYNE2 are associated with p21 expression and SYNE2 variants predict post-operative clinical outcomes in HBV-related hepatocellular carcinoma
Scientific Reports (2016)
-
Anti-cancer effects of p21WAF1/CIP1 transcriptional activation induced by dsRNAs in human hepatocellular carcinoma cell lines
Acta Pharmacologica Sinica (2011)
-
DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control
Oncogene (2006)