Abstract
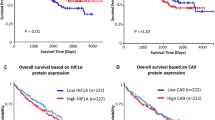
Hypoxia inducible factors HIF1α and HIF2α are important proteins involved in the regulation of the transcription of a variety of genes related to erythropoiesis, glycolysis and angiogenesis. Hypoxic stimulation results in rapid increase of the HIF1α and 2α protein levels, as a consequence of a redox-sensitive stabilization. The HIFαs enter the nucleus, heterodimerize with the HIF1β protein, and bind to DNA at the hypoxia response elements (HREs) of target genes. In this study we evaluated the immunohistochemical expression of these proteins in 108 tissue samples from non-small-cell lung cancer (NSCLC) and in normal lung tissues. Both proteins showed a mixed cytoplasmic/nuclear pattern of expression in cancer cells, tumoural vessels and tumour-infiltrating macrophages, as well as in areas of metaplasia, while normal lung components showed negative or very weak cytoplasmic staining. Positive HIF1α and HIF2α expression was noted in 68/108 (62%) and in 54/108 (50%) of cases respectively. Correlation analysis of HIF2α expression with HIF1α expression showed a significant association (P < 0.0001, r = 0.44). A strong association of the expression of both proteins with the angiogenic factors VEGF (P < 0.004), PD-ECGF (P < 0.003) and bFGF (P < 0.04) was noted. HIF1α correlated with the expression of bek-bFGF receptor expression (P = 0.01), while HIF2α was associated with intense VEGF/KDR-activated vascularization (P = 0.002). HIF2α protein was less frequently expressed in cases with a medium microvessel density (MVD); a high rate of expression was noted in cases with both low and high MVD (P = 0.006). Analysis of overall survival showed that HIF2α expression was related to poor outcome (P = 0.008), even in the group of patients with low MVD (P = 0.009). HIF1α expression was marginally associated with poor prognosis (P = 0.08). In multivariate analysis HIF2α expression was an independent prognostic indicator (P = 0.006, t-ratio 2.7). We conclude that HIF1α and HIF2α overexpression is a common event in NSCLC, which is related to the up-regulation of various angiogenic factors and with poor prognosis. Targeting the HIF pathway may prove of importance in the treatment of NSCLC. © 2001 Cancer Research Campaign http://www.bjcancer.com
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH and Semenza GL (2001) Expression of hypoxia-inducible factor-1α: a novel predictive and prognostic parameter in the radiotherapy of orop haryngeal cancer. Cancer Res 61: 2911–2916
Birner P, Schindl M, Obermair A, Plank C, Breitenecker G and Oberhuber G (2000) Overexpression of hypoxia-inducible factor 1α is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res 60: 4693–4696
Blancher C and Harris AL (1998) The molecular basis of the hypoxia response pathway: tumour hypoxia as a therapy target. Cancer Metast Rev 17: 187–194
Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ and van der Wall E (2001) Levels of hypoxia-inducible factor-1α during breast carcinogenesis. J Natl Cancer Inst 93: 309–314
Brekken RA, Huang X, King SW and Thorpe PE (1998) Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 58: 1952–1959
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E and Keshet E (1998) Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM and Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex II stabilize HIF-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem, (May 31).
de-Porter CR, Eeckhout I, Schelfhout AM, Geerts ML and Roels HJ (1994) Keratinocyte induced chemotaxis in the pathogenesis of Paget’s disease of the breast. Histopathology 24: 349–356
Dong Z, Kumar R, Yang X and Fidler IJ (1997) Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88: 801–810
Ebert BL, Gleadle JM, O’Rourke JF, Bartlett SM, Poulton J and Ratcliffe PJ (1996) Isoenzyme specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. J Biochem 313: 809–814
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y and Fujii-Kuriyama Y (1997) A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 94: 4273–4278
Faller DV (1999) Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol 26: 74–84
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G and Semenza GL (1999) Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res 59: 3915–3918
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD and Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4013
Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hart WH and Broder GJ (1988) Oxygen distribution in squamous cell carcinoma metastases and its relationship in outcome of radiation therapy. Int J Rad Oncol Biol Phys 14: 831–838
Giatromanolaki A, Koukourakis M, O’Byrne K, Kaklamanis L, Dicoglou C, Trichia E, Whitehouse R, Harris AL and Gatter KC (1996) Non small cell lung cancer: C-erbB-2 correlates with low angiogenesis and poor prognosis. Anticancer Res 16: 3819–3825
Giatromanolaki A, Koukourakis MI, Kakolyris S, Turley H, O’Byrne K, Scott PAE, Pezzella F, Georgoulias V, Harris AL and Gatter KC (1998) Vascular endothelial growth factor, wild-type p53 and angiogenesis in early operable non-small cell lung cancer. Clin Cancer Res 4: 3017–3024
Giatromanolaki A, Sivridis E, Koukourakis MI, Georgoulias V, Gatter KC and Harris AL (1999) Intratumoral angiogenesis: a new prognostic indicator for stage I endometrial adenocarcinomas?. Oncol Res 11: 205–212
Giatromanolaki A, Koukourakis MI, Sivridis E, O’Byrne K, Cox G, Thorpe PE, Gatter KC and Harris AL (2000) co-expression of MUC1 glycoprotein with multiple angiogenic factors in non-small cell lung cancer suggets co-activation of angiogenic and migratory pathways. Clin Cancer Res 6: 1917–1921
Goldberg MA, Dunning SP and Bunn HF (1998) Regulation of the erythropoietin gene: evidence that oxygen sensor is a heme protein. Science, 1412–1415
Gopfert T, Gess B, Eckardt KU and Kurtz A (1996) Hypoxia signalling in the control of erythropoietin gene expression in rat hepatocytes. J Cell Physiol 168: 354–361
Griffiths L, Dachs GU, Bicknell R, Harris AL and Stratford IJ (1997) The influence of oxygen-tension and pH on the expression of platelet-derived endothelial cell growth factor thymidine phosphorylase in human breast-tumor cells grown in vitro and in vivo. Cancer Res 57: 570–572
Gu YZ, Moran SM, Hogenesch JB, Wartman L and Bradfield CA (1998) Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr 7: 205–213
Huang LE, Arany Z, Livingston DM and Bunn HF (1996) Activation of Hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its a subunit. J Biol Chem 271: 32253–32259
Huang LE, Gu J, Scheau M and Bunn F (1998) Reulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitine-proteasome pathway. Proc Natl Acad Sci USA 95: 7987–7992
Jiang BH, Agani F, Passaniti A and Semenza GL (1997) V-SRC induces expression of hypoxia inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase-1: involvement of HIF-1 in tumor progression. Cancer Res 57: 5328–5335
Koukourakis MI, Giatromanolaki A, O’Byrne K, Comley M, Whitehouse R, Talbot DC, Gatter KC and Harris AL (1997a) Platelet-derived endothelial cell growth factor expression correlates with tumor angiogenesis and prognosis in non-small cell lung cancer. Br J Cancer 4: 477–481
Koukourakis MI, Giatromanolaki A, O’Byrne K, Whitehouse R, Talbot DC, Gatter KC and Harris AL (1997b) Potential role of bcl-2 as a suppressor of tumour angiogenesis in non small cell lung cancer. Int J Cancer 74: 565–570
Koukourakis M, Giatromanolaki A, Kakolyris S, O’Byrne K, Apostolikas T, Skarlatos J, Gatter KC and Harris AL (1998) Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non small cell lung cancer. Impact on neoangiogenesis and survival. British J Cancer 77: 1696–1703
Koukourakis MI, Giatromanolaki A, O’Byrne KJ, Cox J, Krammer B, Gatter KC and Harris AL (2000a) bcl-2 and c-erbB-2 proteins are involved in the regulation of VEGF and of Thymidine phosphorylase angiogenic activity in non-small cell lung cancer. Clin Exp Metast 17: 545–554
Koukourakis MI, Giatromanolaki A, Thorpe PE, Brekken RA, Sivridis E, Kakolyris S, Georgoulias V, Gatter KC and Harris AL (2000b) Prognostic and pathologic comparison of VEGF/KDR activated microvessel density (aMVD) vs. CD31 standard MVD (sMVD) in non-small cell lung cancer. Cancer Res 60: 3088–3095
Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW and Ratclife PJ (1997) Hypoxia-inducible factor 1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 94: 8104–8109
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275
Mazure NM, Chen EY, Laderoute KR and Giaccia AJ (1999) Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Haras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90: 3322–3331
Norris ML and Millhorn DE (1995) Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem 270: 23774–23779
O’Toole EA, Marinkovich MP, Peavey CL, Amieva MR, Furthmayr H, Mustoe TA and Woodley DT (1997) Hypoxia increases human keratinocyte motility on connective tissue. J Clin Invest 100: 2881–2891
Palmer LA, Semenza GL, Stoler MH and Johns RA (1998) Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol 274: 212–219
Semenza GL and Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454
Semenza GL, Nejfelt MK, Chi SM and Antonarakis SE (1991) Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA 88: 5680–5684
Takagi H, King GL and Aiello LP (1998) Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 47: 1480–1488
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ and Harris AL (2000) The expression and distribution of the hypoxia inducible factors HIF-1á and HIF-2a in normal human tissues, cancers and tumor associated macrophages. Am J Pathol 157: 2411–2421
Tian H, McKnight L and Russel DW (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82
Tungekar MF, Gatter KC, Dunnill MS and Mason DY (1991) Ki-67 immunostaining and survival in operable lung cancer. Histopathology 19: 545–550
Volm M and Koomagi R (2001) Hypoxia-inducible factor (HIF-1) and its relationship to apoptosis and proliferation in lung cancer. Anticancer Res 20: 1527–1533
Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ and Maxwell PH (1998) Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92: 2260–2268
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW and Semenza GI (2000) Expression of hypoxia-inducible factor 1alpha in brain tumours: association with angiogenesis, invasion, and progression. Cancer 88: 2606–2628
Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons JW (1999) Overexpression of Hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res 59: 5830–5835
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D and Giaccia AJ (2000) Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 14: 391–396
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Giatromanolaki, A., Koukourakis, M., Sivridis, E. et al. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 85, 881–890 (2001). https://doi.org/10.1054/bjoc.2001.2018
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2001.2018
Keywords
This article is cited by
-
Hypoxia-inducible factor-2α and its missense mutations: potential role in HCC diagnosis, progression, and prognosis and underlying mechanism
Oncology and Translational Medicine (2022)
-
Hypoxia-sensitive long noncoding RNA CASC15 promotes lung tumorigenesis by regulating the SOX4/β-catenin axis
Journal of Experimental & Clinical Cancer Research (2021)
-
Evolutionary Dynamics in Vascularised Tumours under Chemotherapy: Mathematical Modelling, Asymptotic Analysis and Numerical Simulations
Vietnam Journal of Mathematics (2021)
-
Neddylation blockade induces HIF-1α driven cancer cell migration via upregulation of ZEB1
Scientific Reports (2020)