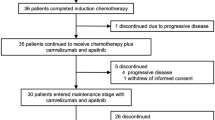
Abstract
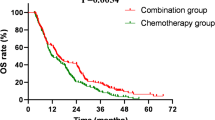
The European Lung Cancer Working Party (ELCWP) designed a 3-arm phase III randomised trial to determine the role of accelerated chemotherapy in extensive-disease (ED) small-cell lung cancer (SCLC). Eligible patients were randomised between the 3 following arms: (A) Standard chemotherapy with 6 courses of EVI (epirubicin 60 mg m–2, vindesine 3 mg m–2, ifosfamide 5 g m–2; all drugs given on day 1 repeated every three weeks. (B) Accelerated chemotherapy with EVI administered every 2 weeks and GM-CSF support. (C) Accelerated chemotherapy with EVI and oral antibiotics (cotrimoxazole). Primary endpoint was survival. 233 eligible patients were randomised. Chemotherapy could be significantly accelerated in arm B with increased absolute dose-intensity. Best response rates, in the population of evaluable patients, were, respectively for arm A, B and C, 59%, 76% and 70%. The response rate was significantly higher in arm B in comparison to arm A (P = 0.04). There was, however, no survival difference with respective median duration and 2-year rate of 286 days and 5% for arm A, 264 days and 6% for arm B and 264 days and 6% for arm C. Severe thrombopenia occurred more frequently in arm B but without an increased rate of bleeding. Non-severe infections were more frequent in arm B and severe infections were less frequent in arm C. Our trial failed to demonstrate, in ED-SCLC, a survival benefit of chemotherapy acceleration by using GM-CSF support. © 2001 Cancer Research Campaign http://www.bjcancer.com
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arriagada R, Le Chevalier T, Pignon JP, Riviere A, Monnet I, Chomy P, Tuchais C, Tarayre M and Ruffie P (1993) Initial chemotherapeutic doses and survival in patients with limited small-cell lung cancer. N Engl J Med 329: 1848–1852
Bunn PAJ, Crowley J, Kelly K, Hazuka MB, Beasley K, Upchurch C, Livingston R, Weiss GR, Hicks WJ and Gandara DR (1995) Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group [published erratum appears in J Clin Oncol 1995 Nov; 13(11): 2860]. J Clin Oncol 13: 1632–1641
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V and Rausch G (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325: 164–170
de Jongh CA, Wade JC, Finley RS, Joshi JH, Aisner J, Wiernik PH and Schimpff SC (1983) Trimethoprim/sulfamethoxazole versus placebo: a double-blind comparison of infection prophylaxis in patients with small cell carcinoma of the lung. J Clin Oncol 1: 302–307
Figueredo AT, Hryniuk WM, Strautmanis I, Frank G and Rendell S (1985) Co-trimoxazole prophylaxis during high-dose chemotherapy of small-cell lung cancer. J Clin Oncol 3: 54–64
Freedman LS (1982) Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1: 121–129
Freedman LS and White SJ (1976) On the use of Pocock and Simon’s method for balancing treatment numbers over prognostic factors in the controlled clinical trial. Biometrics 32: 691–694
Hamm J, Schiller JH, Cuffie C, Oken M, Fisher RI, Shepherd F and Kaiser G (1994) Doseranging study of recombinant human granulocyte-macrophage colony-stimulating factor in small-cell lung carcinoma. J Clin Oncol 12: 2667–2676
Johnson DH (1999) Management of small cell lung cancer: current state of the art. Chest 116: 525S–530S
Lieschke GJ and Burgess AW (1992a) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med 327: 28–35
Lieschke GJ and Burgess AW (1992b) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med 327: 99–106
Luce S, Paesmans M, Berghmans T, Castaigne C, Sotiriou C, Vermylen P and Sculier JP (1998) Revue critique des études randomisées évaluant le rôle de la radiothérapie thoracique adjuvante à la chimiothérapie dans le traitement du cancer bronchique à petites cellules au stade limité. Rev Mal Respir 15: 633–641
Mascaux C, Paesmans M, Berghmans T, Branle F, Lafitte JJ, Lemaitre F, Meert AP, Vermylen P and Sculier JP (2000) A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and metaanalysis. Lung Cancer 30: 23–36
Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA, Schiffer CA, Smith TJ, Somlo G, Wade JC, Wade JL, III Winn RJ, Wozniak AJ and Somerfield MR (2000) 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 18: 3558–3585
Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem O, Mommen P, Ninane V and Klastersky J (2000) Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer 89: 523–533
Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS and Lebeau B (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327: 1618–1624
Pujol JL, Douillard JY, Riviere A, Quoix E, Lagrange JL, Berthaud P, Bardonnet-Comte M, Polin V, Gautier V, Milleron B, Chomy F, Chomy P, Spaeth D and Le Chevalier T (1997) Dose-intensity of a four-drug chemotherapy regimen with or without recombinant human granulocyte-macrophage colony-stimulating factor in extensive-stage small-cell lung cancer: a multicenter randomized phase III study. J Clin Oncol 15: 2082–2089
Sculier JP and Klastersky J (1989) High-dose chemotherapy of small-cell lung cancer with and without bone marrow transplantation. In Basic and clinical concepts of lung cancer, Hansen HH (ed) pp 259–274. Kluwer Academic Publishers: Boston
Sculier JP, Klastersky J, Libert P, Ravez P, Thiriaux J, Lecomte J, Bureau G, Vandermoten G, Dabouis G and Michel J (1990) A randomized study comparing etoposide and vindesine with or without cisplatin as induction therapy for small cell lung cancer. EORTC Lung Cancer Working Party. Ann Oncol 1: 128–133
Sculier JP, Paesmans M, Bureau G, Dabouis G, Libert P, Vandermoten G, Van Cutsem O, Berchier MC, Ries F and Michel J (1993) Multiple-drug weekly chemotherapy versus standard combination regimen in small-cell lung cancer: a phase III randomized study conducted by the European Lung Cancer Working Party. J Clin Oncol 11: 1858–1865
Sculier JP, Paesmans M, Bureau G, Giner V, Lecomte J, Michel J, Berchier MC, Van Cutsem O, Kustner U, Kroll F, Sergysels R, Mommen P and Klastersky J (1996) Randomized trial comparing induction chemotherapy versus induction chemotherapy followed by maintenance chemotherapy in small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol 14: 2337–2344
Sculier JP, Berghmans T, Castaigne C, Luce S, Sotiriou C, Vermylen P and Paesmans M (1998) Maintenance chemotherapy for small cell lung cancer: a critical review of the literature. Lung Cancer 19: 141–151
Steward WP, von Pawel J, Gatzemeier U, Woll P, Thatcher N, Koschel G, Clancy L, Verweij J, de Wit R, Pfeifer W, Fennelly J, von Eiff M and Frisch J (1998) Effects of granulocyte-macrophage colony-stimulating factor and dose intensification of V-ICE chemotherapy in small-cell lung cancer: a prospective randomized study of 300 patients. J Clin Oncol 16: 642–650
Thatcher N (1992) New perspectives in lung cancer. 4. Haematopoietic growth factors and lung cancer treatment. Thorax 47: 119–126
Thatcher N, Girling DJ, Hopwood P, Sambrook RJ, Qian W and Stephens RJ (2000) Improving survival without reducing quality of life in small-cell lung cancer patients by increasing the dose-intensity of chemotherapy with granulocyte colony-stimulating factor support: results of a British Medical Research Council Multicenter Randomized Trial. Medical Research Council Lung Cancer Working Party. J Clin Oncol 18: 395–404
Trillet-Lenoir V, Green J, Manegold C, von Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G and Tomita D (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 29A: 319–324
Trillet-Lenoir V, Green JA, Manegold C, von Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G and Matcham J (1995) Recombinant granulocyte colony stimulating factor in the treatment of small cell lung cancer: a long-term follow-up. Eur J Cancer 31A: 2115–2116
Warde P and Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10: 890–895
Author information
Authors and Affiliations
Consortia
Additional information
The following institutions participated in the trial: Institut Jules Bordet (JP Sculier, M Paesmans, T Berghmans, P Mommen, J Klastersky,), Brussels, Belgium; Hôpital Saint Pierre (R Sergijsels, V Ninane), Brussels, Belgium; CHU de Charleroi (J Thiriaux, J Lecomte), Charleroi, Belgium; Clinique Saint Luc (O Van Cutsem), Namur, Belgium; CHU de Lille, Hôpital Calmette (JJ Lafitte), Lille, France; Hellenic Cancer Institute (A Efremidis, G Koumakis), Athens, Greece; CH de Douai (MC Florin, E Maetz), Douai, France; CH de Tivoli (J Michel), Tivoli, Belgium; Hospital de Sagunto (V Giner Marco), Valencia, Spain; Hôpital d’Hayange (MC Berchier), Hayange, France; CH de Roubaix (F Kroll), Roubaix, France; CHUA Vésale (D Brohée), Montignies-le-Tilleul, Belgium; CHI de Montfermeil (C Zacharias), Montfermeil, France; IMC Mutualités Socialistes (A Tagnon), Tournai, Belgium; Groupe Médical St Rémi (G Bureau), Reims, France; Hôpital de Warquingnies (P Libert, M Richez), Warquignies, Belgium; CH. de Mons (P Recloux), Mons, Belgium; Clinique de la Louvière (F Fortin), Lille, France; CH Dr Schaffner (J Amourette), Lens, France; Clinique Louis Caty (V Richard), Baudour, Belgium; CH du Pays d’Ath (P Ravez), Ath, Belgium; Klinika radiotherapie a onkologie (J Baumöhl), Kosice, Slovakia; CH de Tourcoing (X Ficheroulle), Tourcoing, France; Cabinet de Pneumologie (Y Watrigant), Tourcoing, France; Hôpital Duchenne (JL Crepin), Boulogne-sur-Mer, France; Hôpital Brugmann (A Drowart), Brussels, Belgium; Hôpital de Braine l’Alleud (C Finet), Braine L’Alleud, Belgium.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sculier, J., Paesmans, M., Lecomte, J. et al. A three-arm phase III randomised trial assessing, in patients with extensive-disease small-cell lung cancer, accelerated chemotherapy with support of haematological growth factor or oral antibiotics. Br J Cancer 85, 1444–1451 (2001). https://doi.org/10.1054/bjoc.2001.2114
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2001.2114