Abstract
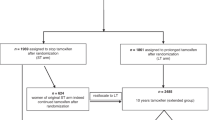
The Breast Cancer Prevention Trial (BCPT-P-1) demonstrated that tamoxifen could reduce the risk of invasive breast cancer in high-risk women by 49%, but that it could also increase the risk of endometrial cancer, vascular events and cataracts. This paper provides an estimate of the net health impacts of tamoxifen administration on high-risk Canadian women with no prior history of breast cancer. The results of the BCPT-P-1 were incorporated into the breast cancer and other modules of Statistics Canada's microsimulation POpulation HEalth Model (POHEM). While the main intervention scenario conformed as closely as possible to the eligibility criteria for tamoxifen in the BCPT-P-1 protocol, 3 additional scenarios were simulated. Predicted absolute risks of breast cancer at 5 years of 1.66%, 3.32% and 4.15% were calculated for women 35 to 70 years of age. When the BCPT-P-1 results were incorporated into the simulation model, the analysis suggests no increase in life expectancy in this risk group. Tamoxifen appeared to be beneficial for women with a 5-year predicted risk of 3.32% or greater. The results of these simulations are particularly sensitive to the reduction in mortality observed in the BCPT-P-1, as well as being sensitive to other characteristics of the simulation model. Overall, the analysis raises questions about the use of tamoxifen in otherwise healthy women at high risk of breast cancer. © 2001 Cancer Research Campaign http://www.bjcancer.com
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bergman L, Beelan MLR, Gallee MPW, Hollema H, Benraadt J and Leeuwen FE and the Comprehensive Cancer Centres' ALERT Group (2000) Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Lancet 356: 881–887
Berthelot J-M, Le Petit C and Flanagan W (1997) Use of longitudinal data in health policy simulation models, American Statistical Association Proceedings, California: 120–129
Bruzzi P (1998) Tamoxifen for the prevention of breast cancer. BMJ, (Editorial)316: 1181–1182
Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J and Wieand HSI (1999) Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 91, (18):1541–1548
Cummings SR, Norton L and Eckert S (1998) Raloxifene reduces risk of breast cancer and may decrease the risk of endometrial cancer in postmenopausal women. Two-year findings from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial. Proc Am Soc Clin Oncol 17:2a, (abstr 3)
Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL and Fisher B (1999) Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 17, (9):2659–2669
Early Breast Cancer Trialists' Collaborative Group (1992) Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. Lancet 339, (8785):71–85
Early Breast Cancer Trialists' Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351: 1451–1467
Emanuel EJ, Wendler D and Grady C (2000) What makes clinical research ethical?. JAMA 283, (20):2701–2711
Evans WK, Will BP, Berthelot J-MB, Logan DM, Mirsky DJ and Kelly N (2000) Breast cancer: better care for less cost: is it possible?. Int J Technol Assess Health Care 16, (4):1168–1178
Fisher B (1999) National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention trial: A reflective commentary. J Natl Cancer Inst 17, (5):632–639
Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, Costantino J, Redmond C, Fisher ER, Bowman DM, Deschênes L, Dimitrov NV, Margolose RG, Robidoux A, Shibata H, Terz J, Paterson AHG, Feldman MI, Farrar W, Evans J and Lickley HL (1996) Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 88, (21):1529–1541
Fisher B, Costantino J, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tn-Chiu E, Ford L and Wolmark N other National Surgical Adjuvant Breast and Bowel Project Investigators (1998) Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 90, (18):1371–1388
Fisher B (2000) Correspondence. J Natl Cancer Inst 92, (8):659
Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsoeur K and Vogel V (1999) Weighing the risks and benefits of tamoxifen treatment for treating breast cancer. J Natl Cancer Inst 91, (21):1829–1846
Jordan VC (1990) Tamoxifen for the prevention of breast cancer. In: De Vita VT, Hellman S and Rosenberg SA editors. Cancer Prevention. Philadelphia (PA),Lippincott; 1–12
Jordan VC (1995) Tamoxifen: toxicities and drug resistance during the treatment and prevention of breast cancer. Annu Rev Pharmacol Toxicol 35: 195–211
Lippman SM and Brown PH (1999) Tamoxifen prevention of breast cancer: an instance of the fingerpost. J Natl Cancer Inst 91(21): 1809–1818, Lippmann SM and BrownPH (2000) Correspondence. JNCI 92, (8):658
Love RR, Wiebe DA, Feyzi JM, Newcomb PA and Chappell RJ (1991) Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med 115: 860–864
National Cancer Institute (May 25, 1999) Press release regarding the Study of Tamoxifen and Raloxifene (STAR) trial. http://cancertrials.nci.nih.gov/NCICANCERTRIALS/zones/Triallnfo/News/star/starpr.asp
Noe LL, Becker RVIII, Gradishar WJ, Gore M and Trotter JP (1999) The cost effectiveness of tamoxifen in the prevention of breast cancer. Am J Manag Care 5, (6):S389–406
Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, Tidy A, Viggers J and Davey J (1998) Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352: 98–101
Pritchard K (1998) Is tamoxifen effective in prevention of breast cancer?. Lancet, Commentary352: 80–81
Radmacher MD and Simon R (2000) Estimation of tamoxifen's efficacy for preventing the formation and growth of breast tumors. J Natl Cancer Inst 92, (1):48–53
Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A and Edwards BK (1997). SEER Cancer Statistics Review: 1973–1994, National Cancer Institute. NIH. Pub No. 97–2789.: Bethesda, MD
Rockhill B, Colditz G and Kaye J (2000) Comment. J Natl Cancer Inst 92, (8):657–658
Smith TJ and Hillner BE (2000) Tamoxifen should be cost-effective in reducing breast cancer risk in high-risk women. J Clin Oncology 18, (2):284–286
Tomas E, Kauppila A, Blanco G, Apaja-Sarkkinen M and Laatikainen T (1995) Comparison between the effects of tamoxifen and toremifene on the uterus in postmenopausal breast cancer patients. Gynecol Oncol 59, (2):261–266
Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, Rotmensz N and Boyle P (1998) Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Lancet 352: 93–97
Will BP, Berthelot J-M, Houle C, Tomiak EM, Verma S and Evans WK (June 1998) The economic impact of locoregional radiotherapy (LRRT) on all post-surgical stage II breast cancer patients in Canada. Poster presentation – International Society for Technology Assessment in Health Care – Ottawa,
Will BP, Le Petit C, Berthelot J-M, Tomiak EM, Verma S and Evans WK (1999) Diagnostic and therapeutic approaches for non-metastatic breast cancer in Canada and their associated costs. Br J Cancer, (9/10):1428–1436
Will BP, Berthelot J-M, Le Petit C, Tomiak EM, Verma S and Evans WK (2000) Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer 36: 724–735
Wolfson MC (1994) POHEM – a framework for understanding and modelling the health of human populations. Wld Hlth Statist Quart 47: 157–176
Zeneca DE (1998) Tamoxifen prescribing information (package insert.) Wilmington.
Abbott RD and McGee D (1987) The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 37: The probability of developing certain cardiovascular diseases in eight years at specified values of some characteristics. U.S. Department of Health and Human Services, NIH Publication 87–2284
Cronin K, Legler JM and Etzioni R (1998) Assessing uncertainty in microsimulation modelling with application to cancer screening interventions. Statist Med 17: 2509–2523
Flanagan W, Berthelot J-MB and Le Petit C (1997) Should long-term hormone replacement therapy in postmenopausal women be promoted?. Conference Proceedings, Canadian Health Economics Research Association
Gentleman J, Robertson D and Tomiak M (1990) Smoothing procedures for simulated longitudinal microdata. Analytical Studies Branch Research Paper Series Number 32, Ottawa, Statistics Canada,
Health and Welfare Canada (1981) The health of Canadians: report of the Canada Health Survey. Statistics Canada, Catalogue 82–538E,
Ma J, Ackerman E and Yang J-J (1993) Parameter sensitivity of a model of viral epidemics simulated with Monte Carlo techniques. 1. Illness attack rates. Int J Biomed Comput 32: 237–253
Miller AB, Baines CJ, To T and Wall C (1992) Canadian National Breast Screening Study 1: Breast cancer detection and death rates among women aged 40 to 49 years. Can Med Assoc J 147: 1459–1476
National Cancer Institute of Canada (1995). Canadian Cancer Statistics 1995. Toronto, Canada
Roos LL, Nicol JP and Cageorge SM (1987) Using administrative data for longitudinal research: comparisons with primary data collection. Journal of Chronic Diseases 40, (1):41–49
Roos LL and Nicol JP (1999) A research registry: uses, development, and accuracy. J Clin Epidemiol 52: 39–47
U.S. Congress, Office of Technology Assessment (1995) Effectiveness and costs of osteoporosis screening and hormone replacement therapy, Washington DC, U.S. Government Printing Office, 1995–387–789: 47402
Weinstein MC, Coxson PG, Williams LW, Pass TM, Stason WB and Goldman L (1987) Forecasting coronary heart disease incidence, mortality, and cost: The Coronary Heart Disease Policy Model. Am J Public Health 77, (11):1417–1426
Will BP, Le Petit C, Berthelot J-M, Tomiak EM, Verma S and Evans WK (1999) Diagnostic and therapeutic approaches for non-metastatic breast cancer in Canada and their associated costs. Br J Cancer, (9/10):1428–1436
Will BP, Berthelot J-M, Le Petit C, Tomiak EM, Verma S and Evans WK (2000) Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer 36: 724–735
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Will, B., Nobrega, K., Berthelot, JM. et al. First do no harm: extending the debate on the provision of preventive tamoxifen. Br J Cancer 85, 1280–1288 (2001). https://doi.org/10.1054/bjoc.2001.2125
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1054/bjoc.2001.2125
Keywords
This article is cited by
-
The Population Health Model (POHEM): an overview of rationale, methods and applications
Population Health Metrics (2015)
-
Validation of population-based disease simulation models: a review of concepts and methods
BMC Public Health (2010)