Abstract
Objective:
Previous studies suggest that darbepoetin might stimulate erythropoiesis in preterm neonates more effectively if injected subcutaneously (s.c.) than if infused intravenously (i.v.). It has been postulated that this is because very high plasma concentrations after i.v. dosing result in urinary loss of the drug. However, this theory has not been tested systematically, and no direct comparisons have been made between s.c. and i.v. dosing of darbepoetin in preterm neonates.
Study design:
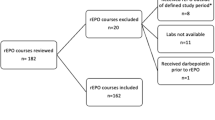
Preterm neonates were eligible for this pilot study if they were born at ⩽32 weeks gestation with a weight of ⩽1500 g, and had a hemoglobin ⩽10.5 g/dl. The darbepoetin was given (4 μg/kg) i.v., over 4 h, if an i.v. was already in place and s.c. if no i.v. was in place. Urine was collected for drug quantification before dosing and for 48 h after. Blood was obtained for immature reticulocyte fraction (IRF), absolute reticulocyte count (ARC) and reticulocyte % before and 96 h after dosing.
Results:
Ten preterm neonates were studied: five received i.v. and five received s.c. darbepoetin. No adverse effects of the administrations were detected. IRF, ARC and reticulocyte % increased in the i.v. and s.c. recipients, and no difference in magnitude of increase was apparent between the groups. Before the darbepoetin was administered, none of the patients had any erythropoietin (Epo) detected in their urine. After i.v. dosing, no darbepoetin was detected in any of the samples, on any of the subjects, over the subsequent 48 h. After s.c. dosing, three of the patients had minimal urinary Epo detected. The patient with the largest urinary loss of drug had only 0.13% of the administered dose detected in the urine. Thus, essentially no urinary loss of drug was observed following either i.v. or s.c. darbepoetin dosing.
Conclusion:
Darbepoetin loss into the urine was below detectable limits among seven patients, while three had minimally detectable urinary losses. i.v. and s.c. dosing resulted in approximately equivalent increases in reticulocyte response when measured 96 h after dosing. On this basis, we speculate that if a patient who is to receive darbepoetin has an i.v. in place, the drug can be given i.v., thus avoiding any discomfort associated with an s.c. injection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA . Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 2003; 31: 290–299.
Cersosimo RJ, Jacobson DR . Epoietin alfa versus darbepoetin alfa in chemotherapy-related anemia. Ann Pharmacother 2006; 40: 58–65.
Warwood TL, Ohls RK, Wiedmeier SE, Lambert DK, Jones C, Scoffield SH et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol 2005; 25: 725–730.
Warwood TL, Ohls RK, Lambert DK, Jones C, Scoffield SH, Gupta N et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol 2006; 26: 296–300.
McIntosh S . Erythropoietin excretion in the premature infant. J Pediatr 1975; 86: 202–206.
Buhrer C, Obladen M, Maier R, Muller C . Urinary losses of recombinant erythropoietin in preterm infants. J Pediatr 2003; 142: 452–453 (letter).
Davis BH, Ornvold K, Bigelow NC . Flow cytometric reticulocyte maturity index: a useful laboratory parameter of erythropoietic activity in anemia. Cytometry 1995; 22: 35–39.
Jelkmann W . The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur J Haematol 2002; 69: 265–274.
Acknowledgements
We thank the NICU nurses at the McKay-Dee NICU for their valuable assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warwood, T., Ohls, R., Lambert, D. et al. Urinary excretion of darbepoetin after intravenous vs subcutaneous administration to preterm neonates. J Perinatol 26, 636–639 (2006). https://doi.org/10.1038/sj.jp.7211596
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.jp.7211596
Keywords
This article is cited by
-
Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates?
Journal of Perinatology (2015)
-
Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial
Pediatric Research (2015)
-
Estimating the nucleated red blood cell ‘emergence time’ in neonates
Journal of Perinatology (2014)
-
Very low birth weight infants qualifying for a ‘late’ erythrocyte transfusion: Does giving darbepoetin along with the transfusion counteract the transfusion's erythropoietic suppression?
Journal of Perinatology (2011)