ABSTRACT
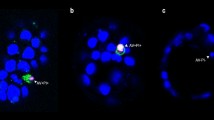
Expression of the adhesion molecules, ICAM-1, VCAM-1, NCAM, CD44, CD49d (VLA-4, α chain), and CD11a (LFA-1, α chain) on mouse oocytes, and pre- and peri-implantation stage embryos was examined by quantitative indirect immunofluorescence microscopy. ICAM-1 was most strongly expressed at the oocyte stage, gradually declining almost to undetectable levels by the expanded blastocyst stage. NCAM, also expressed maximally on the oocyte, declined to undetectable levels beyond the morula stage. On the other hand, CD44 declined from highest expression at the oocyte stage to show a second maximum at the compacted 8-cell/morula. This molecule exhibited high expression around contact areas between trophectoderm and zona pellucida during blastocyst hatching. CD49d was highly expressed in the oocyte, remained significantly expressed throughout and after blastocyst hatching was expressed on the polar trophectoderm. Like CD44, CD49d declined to undetectable levels at the blastocyst outgrowth stage. Expression of both VCAM-1 and CD11a was undetectable throughout. The diametrical temporal expression pattern of ICAM-1 and NCAM compared to CD44 and CD49d suggest that dynamic changes in expression of adhesion molecules may be important for interaction of the embryo with the maternal cellular environment as well as for continuing development and survival of the early embryo.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Turner ML . Cell adhesion molecules: A unifying approach to topographic biology. Biol Rev 1992; 67:359–77.
Hynes RO, Lander AD . Contact and adhesion specificities in the association, migration, and targeting of cells and axons. Cell 1992; 68:303–22.
Springer TA . Adhesion receptors of the immune system. Nature 1990; 346:425–34.
Damsky C, Sutherland A, Fisher S . Extracellular matrix 5: Adhesive interactions in early mammalian embryogenesis, implantation and placentation. FASEB J 1993; 7:1320–9.
Edelman GM . Expression of cell adhesion molecules during embryogenesis and regeneration. Exp Cell Res 1984; 161:3–16.
Takeichi M . Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991; 251:1451–5.
Sutherland AE, Calarco PG, Damsky CH . Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 1993; 119:1175–86.
Almeida EAC, Huovila APJ, Sutherland AE, et al. Mouse egg integrin alpha-6-beta-1 function as a sperm receptor. Cell 1995; 81:1095–104.
Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD . Cell adhesion molecules on the oocyte and preimplantation human embryo. Human Reproduction 1995; 10:1571–78.
Ruck P, Marzusch K, Kaiserling E, et al. Distribution of cell adhesion molecules in decidua of early human pregnancy. Lab Invest 1994; 71:94–101.
Campbell S, Swann HR, Aplin JD, et al. CD44 is expressed throughout pre-implantation human embryo development. Human Reproduction 1995; 10:425–30.
Tian L, Catt JW, O'Neill C, King NJ . Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells. Biol. Reprod 1997; 57:561–8.
Quinn P, Kerin JF, Warnes GM . Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertility and Sterility 1985; 44:439–98.
Quinn P, Warnes GM, Kerin JF, Kirby C . Culture factors affecting the success rate of in votro fertilization and embryo transfer. Ann NY Acad Sci 1985; 442:195–9.
Conover JC, Gwakin RBL . Pre-loading of mouse oocytes with DNA-specific fluorochrome (hoechst 33342) permits rapid detection of sperm-oocyte fusion. J Reprod Fert 1988; 82:681–90.
Johnson GD . Fading of immunofluorescence during microscopy. A study of the phenomenon and its remedy. J Immunol Meth 1982; 55:231–42.
Marlin SD, Springer TA . Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 1987; 51:813–9.
Sligh JEJ, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule-1. Proc Natl Acad Sci USA 1993; 90:8529–33.
Kimber SJ, Bentley J, Ciemerych M, Moller CJ, Bock E . Expression of ncam in fertilized pre- and peri-implantation and parthenogenetically activated mouse embryos. Eur J Cell Biol 1994; 63:102–13.
Xu H, Gonzalo JA, Pierre YS, et al. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med 1994; 180:95–109.
Cremer H, Lange R, Christoph A, et al. Inactivation of the ncam gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994; 367:455–9.
Gurtner GC, Davis V, Li H, et al. Targeted disruption of the murine vcam1 gene: Essential role of VCAM-1 in chorioallantoic fusion and placentatation. Genes & Development 1995; 9:1–14.
Baldwin TJ, Fazeli MS, Doherty P, Walsh FS . Elucidation of the molecular actions of ncam and structurally related cell adhesion molecules. J Cell Biochem 1996; 61:502–13.
Bowen JA, Hunt JS . Expression of cell adhesion molecules in murine placentas and a placental cell line. Biol Reprod 1999; 60:428–34.
Downs KM . Early placental ontogeny in the mouse. Placenta 2002; 23:116–31.
Yang JT, Rayburn H, Hynes RO . Embryonic mesodermal defects in alpha-5 integrin-deficient mice. Cell 1993; 119:1093–105.
Watt FM, Hodivala KJ . Fibronectin and integrin knockouts come unstuck. Curr Biol 1994; 4:270–72.
Guan J, Hynes RO . Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha-4-beta-1. Cell 1990; 60:53–61.
Hynes RO . Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992; 69:11–25.
Berlin C, Berg EL, Briskin MJ, et al. Alpha-4-beta-7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74:185–95.
Evans JP, Schultz RM, Kopf GS . Mouse sperm-egg plasma membrane interactions: Analysis of roles of egg integrins and the mouse sperm homologue of PH-30 (fertilin) beta. J Cell Sci 1995; 108:3267–78.
Hoshi K, Sato A, Sasaki H, Tsuiki A, Yanagida K . Localization of fibronectin on the surface of human spermatozoa and relation to the sperm-egg interaction. Fertility and Sterility 1994; 61:542–7.
George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO . Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993; 119:1079–91.
Barreiro O, Yanez-Mo M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002; 157:1233–45.
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B . CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61:1303–13.
Miyake K, Underhill CB, Lesley J, Kincade PW . Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med 1990; 172:69–75.
Dandekar P, Aggeler J, Talbot P . Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Human Reproduction 1992; 7:391–8.
Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 1997; 90:2217–33.
Laurent TC, Fraser JR . The properties and turnover of hyaluronan. Ciba Foundation Symposium 1986; 124:9–29.
Fillit HM, Blake M, MacDonald C, McCarty M . Immunogenicity of liposome-bound hyaluronate in mice. J Exp Med 1988; 168:971–82.
Tsukita S, Yonemura S, Tsukita S . ERM proteins: Head-to-tail regulation of actin-plasma membrane interaction. TIBS 1997; 22:53–58.
Lesley J, Hyman R, Kincade PW . CD44 and its interaction with extracellular matrix. Adv Immunol 1993; 54:271–335.
Acknowledgements
This work was supported by grant No. 950175 from the Australian National Health and Medical Research Council. D.P. Lu and L. Tian each made an equivalent contribution to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LU, D., TIAN, L., O' NEILL, C. et al. Regulation of cellular adhesion molecule expression in murine oocytes, peri-implantation and post-implantation embryos. Cell Res 12, 373–383 (2002). https://doi.org/10.1038/sj.cr.7290139
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7290139
Keywords
This article is cited by
-
Hashimoto’s thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice
Reproductive Biology and Endocrinology (2019)
-
Biochemical alterations in the oocyte in support of early embryonic development
Cellular and Molecular Life Sciences (2017)
-
EpCAM is decreased but is still present in uterine epithelial cells during early pregnancy in the rat: potential mechanism for maintenance of mucosal integrity during implantation
Cell and Tissue Research (2015)