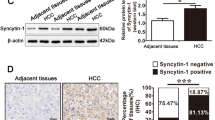
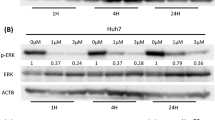
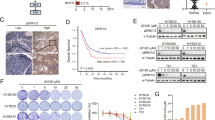
ABSTRACT
Signal transducer and activator of transcription 3 (STAT3) is a recently characterized transcription factor which is essential to liver regeneration. We have previously reported that hepatic stimulator substance (HSS), a novel growth-promoting substance, phosphorylated the epidermal growth factor (EGF) receptors and activated downstream Ras-MAP kinase (extracellular signal-regulated kinases, ERK1/2) cascade. However, whether HSS signal is related to STAT3 pathway remains unclear. The present study is aiming to explore the regulatory effect of activation of ERK1/2 evoked by HSS on STAT3 phosphorylation and STAT3 signaling. Human hepatoma cell line HepG2 was stably transfected with HSS cDNA and HSS expression was measured by Northern blot. The results showed that the transfection of HSS into HepG2 resulted in remarkable increase in cellular proliferation as compared with the non-transfected cells, and it was further proved that the cellular proliferation in the HSS-transfected cells was related to ERK1/2 activation. Treatment of the cells with 50 μM of PD98059, an ERK1/2 specific upstream inhibitor, resulted in ERK1/2 inactivation completely. Inhibition of ERK1/2 allowed the tyrosine of STAT3 to be phosphorylated in a dose-dependent manner to PD98059. Furthermore, transient transfection of STAT3 mutant (STAT3S727A) into HSS-bearing cells could remarkably reverse the inhibitory effect of ERK1/2 on STAT3 phosphorylation. Based upon these results, it is concluded that ERK1/2 negatively modulates STAT3 phosphorylation and this function is dependent on residual serine-727 (S727) of STAT3.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Michalopoulos GK, DeFrances MC . Liver regeneration. Science 1997; 276:60–6.
Fausto N . Liver regeneration. J Hepatol 2000; 32:19–31.
Ihle JN . STATs: signal transducers and activators of transcription. Cell 1996; 84(3):331–4.
Preface JR . STAT signaling. Oncogene 2000; 19:466–7.
Fausto N, Laird AD, Webber EM . Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995; 9(15):1527–36.
Tomiya T, Ogata I, Yamaoka M, et al. The mitogenic activity of hepatocyte growth factor on rat hepatocytes is dependent upon endogenous transforming growth factor-alpha. Am J Pathol 2000; 157(5):1693–701.
Tomiya T, Ogata I, Fujiwara K . Transforming growth factor alpha levels in liver and blood correlate better than hepatocyte growth factor with hepatocyte proliferation during liver regeneration. Am J Pathol 1998; 153(3):955–61.
An W, Liu XJ, Lei TG, Du GG . Growth induction of hepatic stimulator substance in hepatocytes through its regulation on EGF receptors. Cell Res 1999; 9:37–49.
Dai J, An W, Gao DC, Chen L . Influence of hepatic stimulator substance on p21ras expression in human hepatic carcinoma cell BEL-7402. Acta Physiologica Sinica 2000; 52(3):225–9.
Chen L, An W, Tan X, Gao DC, Dai J . Phosphorylation of hepatic stimulator substance on mitogen-activated protein kinase in BEL-7402 hepatoma cells. Chin J Hepatology 2001; 9(1):22–4.
Chen L, Sun H, An W . Production of recombinant hepatic stimulator substance and characterization of its growth promoting activity in hepatoma cells. Hepatology 2002; 36(4):443A.
Cepko C . Introduction of DNA into mammalian cells. In: Ausubel FM, Brent R, Kingston RE, eds. Short Protocols in Molecular Biology, 3rd ed. New York: John Wiley & Sons 1995, 1–49.
Alweine JC, Kemp DJ, Stark GR . Method for detection of specific RNAs in agarose gels by transfer to diabenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci USA 1977; 74:5350–6354.
Goodwin CJ, Holt SJ, Downes S, Marshall NJ . Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J Immunol Methods 1995; 179(1):95–103.
Jian N, Tong Z, Kee WH, Li W, Cao X . Protein kinase C d-associates with and phosphorylates STAT3 in an interleukin-6-dependent manner. J Biol Chem 1999; 274:24392–400.
Bromberg JF, Wrzeszczynska MH, Devgan G, et al. STAT3 as an oncogene. Cell 1999; 98:295–303.
Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985; 150:76–85.
Renart J, Sandoval IV . Western blot. Methods Enzymol 1984; 104:455.
Pelech SL, Sangsera JS . MAP kinase, charting the regulation pathway. Science 1992; 57:1355–6.
Ni Z, Lou W, Leman ES, Gao AC . Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res 2000; 60:1225–8.
Decker T, Kovarik P . Serine phosphorylation of STATs. Oncogene 2000; 19:2628–37.
Leaman DW, Leung S, Li X, Stark GR . Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J 1996; 10(14):1578–88.
Li W, Liang X, Kellendonk C, Poli V, Taub R . STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem 2002; 277(32):28411–7.
Wen Z, Zhong Z, Darnell JE Jr . Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995; 82(2):241–50.
Taga T, Kishimoto T . Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997; 15:797–819.
Kishimoto T, Taga T, Akira S . Cytokine signal transduction. Cell 1994; 76:253–62.
Ihara S, Nakajima K, Fukada T, et al. Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J 1997; 16:5345–52.
Kim H, Hawley TS, Hawley RG, Baumann H . Protein tyrosine phosphatase 2 (SHP-2) moderates signaling by gp130 but is not required for the induction of acute-phase plasma protein genes in hepatic cells. Mol Cell Biol 1998; 18:1525–33.
Jain N, Zhang T, Fong SL, Lim CP, Cao X . Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK). Oncogene 1998; 17(24):3157–67.
Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB . Rapid inhibition of interleukin-6 signaling and STAT3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA 1998; 95:11107–12.
Cheung J, Uchida E, Grammer TC, Blenis J . STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 1997; (11):6508–16.
Ng J, Cantrell D . STAT3 is a serine kinase target in T lym-phocytes. Interleukin 2 and T cell antigen receptor signals converge upon serine 727. J Biol Chem 1997; 272:24542–9.
Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996; 274:1379–83.
Hirano T, Ishihara K, Hibi M . Role of STAT3 in mediating the cell growth, differentiation and survival signals relayed through IL-6 family of cytokine receptor. Oncogene 2000; 19:2548–56.
Acknowledgements
We thank Dr. Toshio Hirano (Department of Molecular Oncology (C7) Graduate School of Medicine, Osaka University, Japan) for the generally gift of STAT3S727A plasmid. We are grateful to Li CHEN for her technical assistance for Western blot. This work was supported by the National Natural Science Foundation of China (No.39870285, No. 0070342).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
TIAN, Z., AN, W. ERK1/2 contributes negative regulation to STAT3 activity in HSS-transfected HepG2 cells. Cell Res 14, 141–147 (2004). https://doi.org/10.1038/sj.cr.7290213
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7290213
Keywords
This article is cited by
-
Liver-derived extracellular vesicles from patients with hepatitis B virus-related acute-on-chronic liver failure impair hepatic regeneration by inhibiting on FGFR2 signaling via miR-218-5p
Hepatology International (2023)
-
ERK1/2-dependent gene expression in the bovine ovulating follicle
Scientific Reports (2018)
-
DCZ3301, a novel cytotoxic agent, inhibits proliferation in diffuse large B-cell lymphoma via the STAT3 pathway
Cell Death & Disease (2017)