ABSTRACT
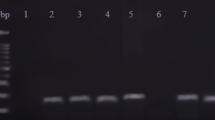
MicroRNAs (miRNAs) are 20-22 nucleotide non-coding RNAs that play important roles in plant and animal development. They are usually processed from larger precursors that can form stem-loop structures. Among 20 miRNA families that are conserved between Arabidopsis and rice, the rice miR395 gene family was unique because it was organized into compact clusters that could be transcribed as one single transcript. We show here that in fact this family had four clusters of total 24 genes. Three of these clusters were segmental duplications. They contained miR395 genes of both 120 bp and 66 bp long. However, only the latter was repeatedly duplicated. The fourth cluster contained miR395 genes of two different sizes that could be the consequences of intergenic recombination of genes from the first three clusters. On each cluster, both 1-duplication and 2-duplication histories were observed based on the sequence similarity between miR395 genes, some of which were nearly identical suggesting a recent origin. This was supported by a miR395 locus survey among several species of the genus Oryza, where two clusters were only found in species with an AA genome, the genome of the cultivated rice. A comparative study of the genomic organization of Medicago truncatula miR395 gene family showed significant expansion of intergenic spaces indicating that the originally clustered genes were drifting away from each other. The diverse genomic organizations of a conserved microRNA gene family in different plant genomes indicated that this important negative gene regulation system has undergone dramatic tune-ups in plant genomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lee RC, Feinbaum RL, Ambros V . The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843–54.
Lau NC, Lim LP, Weinstein EG, Bartel DP . An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001; 294:858–62.
Lai EC, Tomancak P, Williams RW, Rubin GM . Computational identification of Drosophila microRNA genes. Genome Biol 2003; 4:R42.
Bartel B, Bartel DP . MicroRNAs: at the root of plant development? Plant Physiol 2003; 132:709–17.
Jones-Rhoades MW, Bartel DP . Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 2004; 14:787–99.
Palatnik JF, Allen E, Wu X, et al. Control of leaf morphogenesis by microRNAs. Nature 2003; 425:257–63.
Chen X . A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 2004; 303:2022–5.
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP . MicroRNAs in plants. Genes Dev 2002; 16:1616–26.
Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001; 106:23–34.
Lee Y, Jeon K, Lee JT, Kim S, Kim VN . MicroRNA maturation: stepwise processing and subcellular localization. Embo J 2002; 21:4663–70.
Hammond SM, Bernstein E, Beach D, Hannon GJ . An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000; 404:293–6.
Mourelatos Z, Dostie J, Paushkin S, et al. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 2002; 16:720–8.
Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T . New microRNAs from mouse and human. RNA 2003; 9:175–9.
Tanzer A, Stadler PF . Molecular evolution of a microRNA cluster. J Mol Biol 2004; 339:327–35.
Griffiths-Jones S . The microRNA Registry. Nucleic Acids Res 2004; 32 Database issue:D109–11.
Llave C, Xie Z, Kasschau KD, Carrington JC . Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 2002; 297:2053–6.
Park W, Li J, Song R, Messing J, Chen X . CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 2002; 12:1484–95.
Sunkar R, Zhu JK . Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004; 16:2001–19.
Sunkar R, Girke T, Jain PK, Zhu J-K . Cloning and Characterization of MicroRNAs from Rice. Plant Cell 2005; 17:1397–411.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ . Basic local alignment search tool. J Mol Biol 1990; 215:403–10.
Thompson JD, Higgins DG, Gibson TJ . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22:4673–80.
Swofford DL . 4.0 ed. Sinauer Associates, Sunderland, Massachusetts. 2002.
Mathews DH, Sabina J, Zuker M, Turner DH . Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 1999; 288:911–40.
Zuker M . Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31:3406–15.
McCouch SR, Kochert G, Yu ZH, et al. Molecular mapping of rice chromosomes. Theor Appl Genet 1988; 76:815–29.
Elemento O, Gascuel O, Lefranc M-P . Reconstructing the duplication history of tandemly repeated genes. Mol Biol Evol 2002; 19:278–88.
Hass BL, Pires JC, Porter R, Phillips RL, Jackson SA . Comparative genetics at the gene and chromosome levels between rice (Oryza sativa) and wildrice (Zizania palustris). Theor Appl Genet 2003; 107:773–82.
Floyd SK, Bowman JL . Gene regulation: ancient microRNA target sequences in plants. Nature 2004; 428:485–6.
Zhang P, Min W, Li WH . Different age distribution patterns of human, nemotode, and Arabidopsis duplication genes. Genes Dev 2004; 342:263–8.
Mayer K, Murphy G, Tarchini R, et al. Conservation of microstructure between a sequenced region of the genome of rice and multiple segments of the genome of Arabidopsis thaliana. Genome Res 2001; 11:1167–74.
Bancroft I . Duplicate and diverge: the evolution of plant genome microstructure. Trends Genet 2001; 17:89–93.
AGI. Analysis of the genome sequence of the flowering plant Arabisopsis thaliana. Nature 2000; 408:796–815.
Rotte C, Leustek T . Differential subcellular localization and expression of ATP sulfurylase and 5′-adenylylsulfate reductase during ontogenesis of Arabidopsis leaves indicates that cytosolic and plastid forms of ATP sulfurylase may have specialized functions. Plant Physiol 2000; 124:715–24.
Babak T, Zhang W, Morris Q, Blencowe BJ, Hughs TR . Probing microRNAs with microarrays: Tissue specificity and functional inference. RNA 2004; 10:1813–9.
Acknowledgements
We thank Dr. Tom SIMS for comments on the manuscript. Dr. Zhu Kuan CHENG kindly provided the wild rice materials. This work is supported in part by a grant from Northern Illinois University Foundation to Long MAO, and National Institutes of Health (NIH) grant to Mitrick JOHNS and Long MAO (No. 44-G1A62164), and a grant from the National Natural Science Foundation of China for oversea young scholars to Long MAO (No. 30228022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GUDDETI, S., ZHANG, D., LI, A. et al. Molecular evolution of the rice miR395 gene family. Cell Res 15, 631–638 (2005). https://doi.org/10.1038/sj.cr.7290333
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7290333
Keywords
This article is cited by
-
MiRNA: the taskmaster of plant world
Biologia (2021)
-
Novel insights into expansion and functional diversification of MIR169 family in tomato
Planta (2020)
-
In Silico Identification and Validation of Potential microRNAs in Kinnow Mandarin (Citrus reticulata Blanco)
Interdisciplinary Sciences: Computational Life Sciences (2018)
-
Altered expression profiles of microRNA families during de-etiolation of maize and rice leaves
BMC Research Notes (2017)
-
Heterologous expression of a rice miR395 gene in Nicotiana tabacum impairs sulfate homeostasis
Scientific Reports (2016)