Abstract
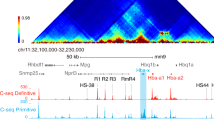
EKLF is an erythroid-specific, zinc finger-containing transcription factor essential for the activation of the mammalian beta globin gene in erythroid cells of definitive lineage. We have prepared a polyclonal anti-mouse EKLF antibody suitable for Western blotting and immunoprecipitation (IP) qualities, and used it to define the expression patterns of the EKLF protein during mouse erythroid development. We have also used this antibody for the chromatin-immunoprecipitation (ChIP) assay. EKLF was found to bind in vivo at both the mouse beta-major-globin promoter and the HS2 site of beta-LCR in the mouse erythroleukemia cells (MEL) in a DMSO-inducible manner. The DMSO-induced bindings of EKLF as well as three other proteins, namely, RNA polymerase II, acetylated histone H3, and methylated histone H3, were not abolished but significantly lowered in CB3, a MEL-derived cell line with null-expression of p45/NF-E2, an erythroid-enriched factor needed for activation of the mammalian globin loci. Interestingly, binding of EKLF in vivo was also detected in the mouse alpha-like globin locus, at the adult alpha globin promoter and its far upstream regulatory element alpha-MRE (HS26). This study provides direct evidence for EKLF-binding in vivo at the major regulatory elements of the mouse beta-like globin gene clusters the data also have interesting implications with respect to the role of EKLF-chromatin interaction in mammalian globin gene regulation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bulger M, Groudine M . Looping versus linking: toward a model for long-distance gene activation. Genes Dev 1999; 13:2465–2477.
Engel JD, Tanimoto K . Looping, linking, and chromatin activity: new insights into beta-globin locus regulation. Cell 2000; 100:499–502.
Li Q, Peterson KR, Fang X, Stamatoyannopoulos G . Locus control regions. Blood 2002; 100:3077–3086.
de Laat W, Grosveld F . Spatial organization of gene expression: the active chromatin hub. Chromosome Res 2003; 11:447–459.
Kingsley PD, Malik J, Emerson RL, et al. “Maturational” globin switching in primary primitive erythroid cells. Blood 2006; 107:1665–1672.
Whitney JB, 3rd . Differential control of the synthesis of two hemoglobin beta chains in normal mice. Cell 1977; 12:863–871.
Andrews NC . The NF-E2 transcription factor. Int J Biochem Cell Biol 1998; 30:429–432.
Martin DI, Tsai SF, Orkin SH . Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature 1989; 338:435–438.
Tsang AP, Visvader JE, Turner CA, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 1997; 90:109–119.
Miller IJ, Bieker JJ . A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol 1993; 13:2776–2786.
Bieker JJ . Isolation, genomic structure, and expression of human erythroid Kruppel-like factor (EKLF). DNA Cell Biol 1996; 15:347–352.
Perkins AC, Sharpe AH, Orkin SH . Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 1995; 375:318–322.
Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F . Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 1995; 375:316–318.
Tewari R, Gillemans N, Wijgerde M, et al. Erythroid Kruppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5'HS3 of the beta-globin locus control region. Embo J 1998; 17:2334–2341.
Gillemans N, Tewari R, Lindeboom F, et al. Altered DNA-binding specificity mutants of EKLF and Sp1 show that EKLF is an activator of the beta-globin locus control region in vivo. Genes Dev 1998; 12:2863–2873.
Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD . Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev 2000; 14:2778–2794.
Wijgerde M, Gribnau J, Trimborn T, et al. The role of EKLF in human beta-globin gene competition. Genes Dev 1996; 10:2894–2902.
Bieker JJ, Southwood CM . The erythroid Kruppel-like factor transactivation domain is a critical component for cell-specific inducibility of a beta-globin promoter. Mol Cell Biol 1995; 15:852–860.
Chen X, Bieker JJ . Unanticipated repression function linked to erythroid Kruppel-like factor. Mol Cell Biol 2001; 21:3118–3125.
Kadam S, McAlpine GS, Phelan ML, et al. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev 2000; 14:2441–2451.
Pandya K, Donze D, Townes TM . Novel transactivation domain in erythroid Kruppel-like factor (EKLF). J Biol Chem 2001; 276:8239–8243.
Bieker JJ . Kruppel-like factors: three fingers in many pies. J Biol Chem 2001; 276:34355–34358.
Pandya K, Townes TM . Basic residues within the Kruppel zinc finger DNA binding domains are the critical nuclear localization determinants of EKLF/KLF-1. J Biol Chem 2002; 277:16304–16312.
Quadrini KJ, Bieker JJ . Kruppel-like zinc fingers bind to nuclear import proteins and are required for efficient nuclear localization of erythroid Kruppel-like factor. J Biol Chem 2002; 277:32243–32252.
Armstrong JA, Bieker JJ, Emerson BM . A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 1998; 95:93–104.
Geller R, Levenson R, Housman D . Significance of the cell cycle in commitment of murine erythroleukemia cells to erythroid differentiation. J Cell Physiol 1978; 95:213–222.
Nudel U, Salmon JE, Terada M, et al. Differential effects of chemical inducers on expression of beta globin genes in murine erythroleukemia cells. Proc Natl Acad Sci USA 1977; 74:1100–1104.
Lu SJ, Rowan S, Bani MR, Ben-David Y . Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc Natl Acad Sci USA 1994; 91:8398–8402.
Shyu YC, Lee TL, Ting CY, et al. Sumoylation of p45/NF-E2: nuclear positioning and transcriptional activation of the mammalian beta-like globin gene locus. Mol Cell Biol 2005; 25:10365–10378.
Hsu CT, Ting CY, Ting CJ, et al. Vaccination against gonadotropin-releasing hormone (GnRH) using toxin receptor-binding domain-conjugated GnRH repeats. Cancer Res 2000; 60:3701–3705.
Liu JJ, Hou SC, Shen C-KJ . Erythroid gene suppression by NF-kappa B. J Biol Chem 2003; 278:19534–19540.
Daftari P, Gavva NR, Shen C-KJ . Distinction between AP1 and NF-E2 factor-binding at specific chromatin regions in mammalian cells. Oncogene 1999; 18:5482–5486.
Sawado T, Igarashi K, Groudine M . Activation of beta-major globin gene transcription is associated with recruitment of NF-E2 to the beta-globin LCR and gene promoter. Proc Natl Acad Sci USA 2001; 98:10226–10231.
Southwood CM, Downs KM, Bieker JJ . Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn 1996; 206:248–259.
Kiekhaefer CM, Grass JA, Johnson KD, Boyer ME, Bresnick EH . Hematopoietic-specific activators establish an overlapping pattern of histone acetylation and methylation within a mammalian chromatin domain. Proc Natl Acad Sci USA 2002; 99:14309–14314.
Brand M, Ranish JA, Kummer NT, et al. Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat Struct Mol Biol 2004; 11:73–80.
Sawado T, Halow J, Bender MA, Groudine M . The beta-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev 2003; 17:1009–1018.
Forsberg EC, Downs KM, Christensen HM, et al. Developmentally dynamic histone acetylation pattern of a tissue-specific chromatin domain. Proc Natl Acad Sci USA 2000; 97:14494–14499.
Johnson KD, Grass JA, Park C, et al. Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain. Mol Cell Biol 2003; 23:6484–6493.
Forsberg EC, Downs KM, Bresnick EH . Direct interaction of NF-E2 with hypersensitive site 2 of the beta-globin locus control region in living cells. Blood 2000; 96:334–339.
Johnson KD, Christensen HM, Zhao B, Bresnick EH . Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol Cell 2001; 8:465–471.
Anguita E, Johnson CA, Wood WG, Turner BM, Higgs DR . Identification of a conserved erythroid specific domain of histone acetylation across the alpha-globin gene cluster. Proc Natl Acad Sci USA 2001; 98:12114–12119.
Anguita E, Hughes J, Heyworth C, et al. Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. Embo J 2004; 23:2841–2852.
Reddy PM, Shen C-KJ . Erythroid differentiation of mouse erythroleukemia cells results in reorganization of protein-DNA complexes in the mouse beta maj globin promoter but not its distal enhancer. Mol Cell Biol 1993; 13:1093–1103.
Francastel C, Magis W, Groudine M . Nuclear relocation of a transactivator subunit precedes target gene activation. Proc Natl Acad Sci USA 2001; 98:12120–12125.
Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 1995; 81:695–704.
Acknowledgements
We thank our lab colleagues for various reagents and for their help. We also appreciate the discussions on the topic with Jim Bieker. This research was supported by a grant from the National Health Research Institute (NHRI), and by the Academia Sinica, Taipei.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shyu, YC., Wen, SC., Lee, TL. et al. Chromatin-binding in vivo of the erythroid kruppel-like factor, EKLF, in the murine globin loci. Cell Res 16, 347–355 (2006). https://doi.org/10.1038/sj.cr.7310045
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7310045
Keywords
This article is cited by
-
An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption
Nature (2023)
-
Direct competition between DNA binding factors highlights the role of Krüppel-like Factor 1 in the erythroid/megakaryocyte switch
Scientific Reports (2017)
-
miR-122-mediated translational repression of PEG10 and its suppression in human hepatocellular carcinoma
Journal of Translational Medicine (2016)
-
Differential regulation of the α-globin locus by Krüppel-like factor 3 in erythroid and non-erythroid cells
BMC Molecular Biology (2014)
-
Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells
Nature Genetics (2010)