Abstract
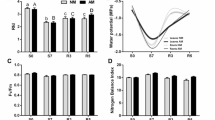
A symptom of chilling injury is development of water deficit in shoots, resulting from an imbalance of water transport and transpiration. In this work, two rice varieties (Oryza sativa L. var. Wasetoitsu and Somewake) seedlings were chilled at 7 °C, followed by recovery at 28 °C. Based on the growth phenotype and electrolyte leakage tests, Somewake was shown to be a chilling-tolerant variety, and Wasetoitsu a chilling-sensitive one. The chilling stress reduced markedly the relative water content (RWC) of leaves, accumulative transpiration and osmotic root hydraulic conductivity (Lp) in both varieties. But when returned to 28 °C, the water relation balance of Somewake recovered better. The mRNA expression profile of all the 11 plasma membrane intrinsic proteins (PIPs), a subgroup of aquaporins, was subsequently determined by real-time reverse transcription (RT)-PCR with TaqMan-minor grove binder (MGB) probes derived from rice var. Nipponbare during chilling treatment and recovery. Most of the PIP genes was down-regulated at the low temperature, and recovered at the warm temperature. The relative expression of some PIPs in both Somewake and Wasetoitsu decreased in parallel during the chilling. However during the recovery, the relative expression of OsPIP1;1, OsPIP2;1, OsPIP2;7 in shoots and OsPIP1;1, OsPIP2;1 in roots were significantly higher in Somewake than Wasetoitsu. This supports the role of PIPs in re-establishing water balance after chilling conditions. We discuss the diversified roles played by members of the aquaporin PIP subfamily in plant chilling tolerance depending on aquaporin isoforms, plant tissue and the stage of chilling duration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Thomashow MF . So what's new in the field of plant cold acclimation? lots! Plant Physiol 2001; 125:89–93.
Sanders P, Markhart AH . Root system functions during chilling temperatures: injury and acclimation. In: Basra S, eds. Crop responses and adaptations to temperature stress. Haworth Press: New York 2001:77–108
Perez de Juan J ., Irigoyen JJ, Sanchez-Diaz M . Chilling of drought-hardened and non-hardened plants of different chilling-sensitive maize lines Changes in water relations and ABA contents. Plant Sci 1997; 122:71–79.
Aroca R, Tognoni F, Irigoyen JJ, Sanchez-Diaz M, Pardossi A . Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem 2001; 39:1067–1073.
Melkonian J, Yu LX, Setter TL . Chilling responses of maize (Zea mays L.) seedlings: root hydraulic conductance, abscisic acid, and stomatal conductance. J Exp Bot 2004; 55:1751–1760.
Aroca R, Amodeo G ., Fernandez-Illescas S, et al. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 2005; 137:341–353.
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M . Identification of 33 Rice Aquaporin genes and analysis of their expression and function. Plant Cell Physiol 2005; 46:1568–1577.
Vernieri P, Lenzi A, Figaro M, Tognoni F, Pardossi A . How the roots contribute to the ability of Phaseolus vulgaris L. to cope with chilling-induced water stress. J Exp Bot 2001; 52:2199–2206.
Fennell A, Markhart AH . Rapid acclimation of root hydraulic conductivity to low temperature. J Exp Bot 1998; 49:879–894.
Wan X, Zwiazek JJ, Lieffers VJ, Landhausser SM . Hydraulic conductance in aspen (Populus tremuloides) seedlings exposed to low root temperatures. Tree Phyiol 2000; 21:691–696.
Lee SH, Singh AP, Chung GC, et al. Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol Plant 2004; 120:413–420.
Lee SH, Chung GC, Steudle E . Gating of aquaporins by low temperature in roots of chilling-sensitive cucumber and chilling-tolerant figleaf gourd. J Exp Bot 2005; 56:985–995.
Javot H, Maurel C . The role of aquaporins in root water uptake. Ann Bot (Lond) 2002; 90:301–313.
Maurel C, Javot H, Lauvergeat V, et al. Molecular physiology of aquaporins in plants. Int Rev Cytol 2002; 215:105–148.
Maurel C, Chrispeels MJ . Aquaporins. A molecular entry into plant water relations. Plant Physiol 2001; 125:135–138.
Tyerman SD, Niemietz CM, Bramley H . Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 2002; 25:173–194.
Steudle E . Water uptake by roots: effects of water deficit. J Exp Bot 2000; 51:1531–1542.
Steudle E, Peterson C . How does water get through roots? J Exp Bot 1998; 49:775–788.
Li L, Li S, Tao Y, Kitagawa Y . Molecular cloning of a novel water channel from rice: its products expression in Xenopus oocytes and involvement in chilling tolerance. Plant Sci 2000; 154:43–51
Jang JY, Kim DG, Kim YO, Kim JS, Kang H . An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol 2004; 54:713–725.
Maurel C . Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 1997; 48:399–429.
Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC . Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 1999; 50:1055–1071.
Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ . From genome to function: the Arabidopsis aquaporins. Genome Biol 2002; 3:Research 0001. Epub 1–7.
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R . Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 2001; 125:1206–1215.
Gachon C, Mingam A, Charrier B . Real-time PCR: what relevance to plant studies? J Exp Bot 2004; 55:1445–1454.
Ni JS . Solution culture of rice. In: Xue YL, eds. Experiment Handbook of Plant Physiology. Shanghai, China. 1985:63–65 (in Chinese)
Lian HL, Yu X, Ye Q, et al. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 2004; 45:481–489.
Miyamoto N, Steudle E, Hirasawa T, Lafitte R . Hydraulic conductivity of rice roots. J Exp Bot 2001; 52:1835–1846.
Martinez-Ballesta MC, Aparicio F, Pallas V, Martinez V, Carvajal M . Influence of saline stress on root hydraulic conductance and PIP expression in Arabidopsis. J Plant Physiol 2003; 160:689–697.
Nishi R, Hashimoto H, Kidou S, Uchimiya H, Kato A . Isolation and characterization of a rice cDNA which encodes a ubiquitin protein and a 52 amino acid extension protein. Plant Mol Biol 1993; 22:159–161.
Ohshima Y, Iwasaki I, Suga S, Murakami M, Inoue K . Maeshima M . Low aquaporin content and low osmotic water permeability of the plasma and vacuolar membranes of a CAM plant Graptopetalum paraguayense: comparison with radish. Plant Cell Physiol 2001; 42:1119–1129.
Bradford MM . A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–254.
Sambrook F, Fritsch EF, Maniatis T . Molecular Cloning: A Laboratory Manual. 2nd edn. Cold Spring Harbor, New York 1989: 1860–1865.
Kitagawa Y, Yoshizaki K . Water stress-induced chilling tolerance in rice; putative relationship between chilling tolerance and Ca2+ flux. Plant Sci 1998; 137:73–85.
Luu DT, Maurel C . Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ 2005; 28:85–96.
Suga S, Murai M, Kuwagata T, Maeshima M . Differences in aquaporin levels among cell types of radish and measurement of osmotic water permeability of individual protoplasts. Plant Cell Physiol 2003; 44:277–286.
Acknowledgements
We thank Prof. Yoshichika Kitagawa (Akita Prefectural University, Japan) for kindly providing the Wasetoitsu and Somewake rice seeds and for reviewing the manuscript. We also thank Dr Weining Sun for her technical assistance and review of the manuscript, and Dr David Lane for help with revision. This work was supported by the Key Basic Research Special Fund of CAS (KSCXZ–SW–116).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, X., Peng, Y., Zhang, M. et al. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res 16, 599–608 (2006). https://doi.org/10.1038/sj.cr.7310077
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7310077
Keywords
This article is cited by
-
Identification of genes associated with the regulation of cold tolerance and the RNA movement in the grafted apple
Scientific Reports (2023)
-
Rice MYB transcription factor OsMYB1R1 negatively regulates drought resistance
Plant Growth Regulation (2023)
-
Comparative transcriptome study of switchgrass (Panicum virgatum L.) homologous autopolyploid and its parental amphidiploid responding to consistent drought stress
Biotechnology for Biofuels (2020)
-
AtHKT1 gene regulating K+ state in whole plant improves salt tolerance in transgenic tobacco plants
Scientific Reports (2018)
-
Breeding approaches and genomics technologies to increase crop yield under low-temperature stress
Plant Cell Reports (2017)