Abstract
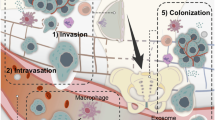
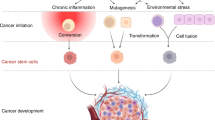
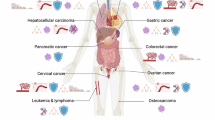
The importance of cancer stem cells (CSCs) in tumor-initiation has been firmly established in leukemia and recently reported for a variety of solid tumors. However, the role of CSCs in multistage cancer progression, particularly with respect to metastasis, has not been well-defined. Cancer metastasis requires the seeding and successful colonization of specialized CSCs at distant organs. The biology of normal stem cells and CSCs share remarkable similarities and may have important implications when applied to the study of cancer metastasis. Furthermore, overlapping sets of molecules and pathways have recently been identified to regulate both stem cell migration and cancer metastasis. These molecules constitute a complex network of cellular interactions that facilitate both the initiation of the pre-metastasis niche by the primary tumor and the formation of a nurturing organ microenvironment for migrating CSCs. In this review, we surveyed the recent advances in this dynamic field and propose a unified model of cancer progression in which CSCs assume a central role in both tumorigenesis and metastasis. Better understanding of CSCs as a fundamental component of the metastatic cascade will lead to novel therapeutic strategies against metastatic cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weigelt B, Peterse JL, van't Veer LJ . Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5:591–602.
Hill RP . Identifying cancer stem cells in solid tumors: case not proven. Cancer Res 2006; 66:1891–1895.
Weissman IL . Stem cells: units of development, units of regeneration, and units in evolution. Cell 2000; 100:157–168.
Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114:763–776.
Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005; 121:823–835.
Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature 2006; 439:84–88.
Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006; 439:993–997.
Wagers AJ, Weissman IL . Plasticity of adult stem cells. Cell 2004; 116:639–648.
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC . Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996; 183:1797–1806.
Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 2000; 287:1804–1808.
Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T . In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol 2004; 6:436–442.
McCulloch EA, Till JE . Perspectives on the properties of stem cells. Nat Med 2005; 11:1026–1028.
Moore KA, Lemischka IR . Stem cells and their niches. Science 2006; 311:1880–1885.
Gat U, DasGupta R, Degenstein L, Fuchs E . De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998; 95:605–614.
Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998; 19:379–383.
Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002; 111:251–263.
Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 2004; 101:266–271.
Lo Celso C, Prowse DM, Watt FM . Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 2004; 131:1787–1799.
Pinto D, Gregorieff A, Begthel H, Clevers H . Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003; 17:1709–1713.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005; 434:843–850.
Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423:409–414.
Taipale J, Cooper MK, Maiti T, Beachy PA . Patched acts catalytically to suppress the activity of smoothened. Nature 2002; 418:892–897.
Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 2000; 6:1278–1281.
Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003; 423:448–452.
Duncan AW, Rattis FM, DiMascio LN, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 2005; 6:314–322.
He XC, Zhang J, Tong WG, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 2004; 36:1117–1121.
Larsson J, Blank U, Helgadottir H, et al. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood 2003; 102:3129–3135.
Li L, Neaves WB . Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006; 66:4553–4557.
Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105:369–377.
Jackson KA, Mi T, Goodell MA . Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999; 96:14482–14486.
Thiery JP . Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003; 15:740–746.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3:730–737.
Al-Hajj M, Clarke MF . Self-renewal and solid tumor stem cells. Oncogene 2004; 23:7274–7282.
Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63:5821–5828.
Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 2003; 100:15178–15183.
Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004; 432:396–401.
Clarke MF, Fuller M . Stem cells and cancer: two faces of eve. Cell 2006; 124:1111–1115.
Huntly BJ, Gilliland DG . Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 2005; 5:311–321.
Polyak K, Hahn WC . Roots and stems: stem cells in cancer. Nat Med 2006; 12:296–300.
Reya T, Morrison SJ, Clarke MF, Weissman IL . Stem cells, cancer, and cancer stem cells. Nature 2001; 414:105–111.
Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA 2000; 97:14720–14725.
Lessard J, Sauvageau G . Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003; 423:255–260.
Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003; 423:302–305.
Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003; 425:962–967.
Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006; 66:6063–6071.
Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 2006; 441:475–482.
Miyamoto T, Weissman IL, Akashi K . AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA 2000; 97:7521–7526.
Welm AL, Kim S, Welm BE, Bishop JM . MET and MYC cooperate in mammary tumorigenesis. Proc Natl Acad Sci USA 2005; 102:4324–4329.
Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science 2004; 306:1568–1571.
Jaiswal S, Traver D, Miyamoto T, et al. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proc Natl Acad Sci USA 2003; 100:10002–10007.
Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 2004; 351:657–667.
Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006; 442:818–822.
Vescovi AL, Galli R, Reynolds BA . Brain tumour stem cells. Nat Rev Cancer 2006; 6:425–436.
Rizvi AZ, Swain JR, Davies PS, et al. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. ProcNatl Acad Sci USA 2006; 103:6321–6325.
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ . Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer 2005; 5:899–904.
Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 2002; 1:269–277.
Schofield R . The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4:7–25.
Li L, Xie T . Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005; 21:605–631.
Scadden DT . The stem-cell niche as an entity of action. Nature 2006; 441:1075–1079.
Xie T, Spradling AC . A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000; 290:328–330.
Lin H . The stem-cell niche theory: lessons from flies. Nat Rev Genet 2002; 3:931–940.
Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT . Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 2001; 294:2542–2545.
Schulz C, Kiger AA, Tazuke SI, et al. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics 2004; 167:707–723.
Song X, Wong MD, Kawase E, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 2004; 131:1353–1364.
Tulina N, Matunis E . Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 2001; 294:2546–2549.
Xie T, Spradling AC . Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 1998; 94:251–260.
Morrison SJ, Kimble J . Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006; 441:1068–1074.
Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425:841–846.
Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425:836–841.
Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005; 106:1232–1239.
Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 2005; 201:1781–1791.
Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF . The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med 2001; 1:621–632.
Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3:537–549.
Campos LS, Decker L, Taylor V, Skarnes W . Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem 2006; 281:5300–5309.
Fuchs E, Tumbar T, Guasch G . Socializing with the neighbors: stem cells and their niche. Cell 2004; 116:769–778.
Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R . Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature 1996; 380:171–175.
Kurata S, Okuyama T, Osada M, et al. p51/p63 controls subunit alpha3 of the major epidermis integrin anchoring the stem cells to the niche. J Biol Chem 2004; 279:50069–50077.
Watt FM, Hogan BL . Out of Eden: stem cells and their niches. Science 2000; 287:1427–1430.
Wilson A, Murphy MJ, Oskarsson T, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev 2004; 18:2747–2763.
Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118:149–161.
Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004; 103:3258–3264.
Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121:1109–1121.
Wagers AJ . Stem cell grand SLAM. Cell 2005; 121:967–970.
Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004; 10:64–71.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116:1195–1201.
Papayannopoulou T . Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood 2004; 103:1580–1585.
Selleri C, Ragno P, Ricci P, et al. The metastasis-associated 67 kDa laminin receptor is involved in G-CSF-induced hematopoietic stem cell mobilization. Blood 2006; 108:2476–2484.
Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC . The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997; 185:111–120.
Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005; 201:1307–1318.
Lapidot T, Kollet O . The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002; 16:1992–2003.
Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283:845–848.
Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL . Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med 2002; 195:1145–1154.
Liotta LA . An attractive force in metastasis. Nature 2001; 410:24–25.
Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50–56.
Murphy PM . Chemokines and the molecular basis of cancer metastasis. N Engl J Med 2001; 345:833–835.
Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK . Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene 2004; 23:157–167.
Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004; 64:8604–8612.
Bernards R . Cancer: cues for migration. Nature 2003; 425:247–248.
Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004; 6:459–469.
Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res 2004; 64:4302–4308.
John A, Tuszynski G . The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 2001; 7:14–23.
Rafii S, Lyden D, Benezra R, Hattori K, Heissig B . Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer 2002; 2:826–835.
Pratap J, Javed A, Languino LR, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol 2005; 25:8581–8591.
Chinni SR, Sivalogan S, Dong Z, et al. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 2006; 66:32–48.
Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002; 109:625–637.
Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML . Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol 2000; 28:1274–1285.
Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest 2003; 112:160–169.
Raubenheimer EJ, Noffke CE . Pathogenesis of bone metastasis: a review. J Oral Pathol Med 2006; 35:129–135.
Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 2006; 439:599–603.
Mihai R, Stevens J, McKinney C, Ibrahim NB . Expression of the calcium receptor in human breast cancer – a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol 2006; 32:511–515.
Sneddon JB, Zhen HH, Montgomery K, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA 2006; 103:14842–14847.
Bernards R, Weinberg RA . A progression puzzle. Nature 2002; 418:823.
Gupta PB, Kuperwasser C, Brunet JP, et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet 2005; 37:1047–1054.
Ramaswamy S, Ross KN, Lander ES, Golub TR . A molecular signature of metastasis in primary solid tumors. Nat Genet 2003; 33:49–54.
Singh D, Febbo PG, Ross K, et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 2002; 1:203–209.
van't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415:530–536.
Paget S . The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989; 8:98–101.
Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005; 436:518–524.
Minn AJ, Kang Y, Serganova I, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005; 115:44–55.
Kang Y . Functional genomic analysis of cancer metastasis: biologic insights and clinical implications. Expert Rev Mol Diagn 2005; 5:385–395.
Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438:820–827.
Fidler IJ, Talmadge JE . Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res 1986; 46:5167–5171.
Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM . Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer 2005; 41:2792–2805.
Muller A, Sonkoly E, Eulert C, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer 2006; 118:2147–2157.
Pathak SK, Sharma RA, Steward WP, et al. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur J Cancer 2005; 41:61–70.
Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006.
Galmozzi E, Facchetti F, La Porta CA . Cancer stem cells and therapeutic perspectives. Curr Med Chem 2006; 13:603–607.
Kawamata H, Tachibana M, Fujimori T, Imai Y . Differentiation-inducing therapy for solid tumors. Curr Pharm Des 2006; 12:379–385.
Dean M, Fojo T, Bates S . Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5:275–284.
Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B . Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA 1992; 89:2804–2808.
Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR . Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood 1991; 77:1218–1227.
Civin CI, Trischmann T, Kadan NS, et al. Highly purified CD34-positive cells reconstitute hematopoiesis. J Clin Oncol 1996; 14:2224–2233.
Hao QL, Smogorzewska EM, Barsky LW, Crooks GM . In vitro identification of single CD34+CD38- cells with both lymphoid and myeloid potential. Blood 1998; 91:4145–4151.
Uchida N, Weissman IL . Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin-Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med 1992; 175:175–184.
Ikuta K, Weissman IL . Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA 1992; 89:1502–1506.
Blanpain C, Fuchs E . Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 2006; 22:339–373.
Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research 2005; 65:9328–9337.
Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 2004; 117:3539–3545.
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ . Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65:10946–10951.
Acknowledgements
We thank members of our laboratories for the helpful discussion of this manuscript. We apologize to our colleagues whose important studies could not be cited directly here owing to space limitations. Our research was supported by American Cancer Society (RSG MGO-110765), Department of Defense (BC051647), Susan G Komen Foundation (BCTR0503765), New Jersey Commission on Cancer Research (05-2408-CCR-E0), National Cancer Institute, the WM Keck Foundation and the Kleberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, F., Tiede, B., Massagué, J. et al. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 17, 3–14 (2007). https://doi.org/10.1038/sj.cr.7310118
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.cr.7310118
Keywords
This article is cited by
-
The clinical significance of CD44v6 in malignant and benign primary bone tumors
BMC Musculoskeletal Disorders (2023)
-
Cancer ego-system in glioma: an iron-replenishing niche network systemically self-organized by cancer stem cells
Inflammation and Regeneration (2022)
-
Establishment of an antibody specific for AMIGO2 improves immunohistochemical evaluation of liver metastases and clinical outcomes in patients with colorectal cancer
Diagnostic Pathology (2022)
-
Small protein complex prediction algorithm based on protein–protein interaction network segmentation
BMC Bioinformatics (2022)
-
AMIGO2 contained in cancer cell-derived extracellular vesicles enhances the adhesion of liver endothelial cells to cancer cells
Scientific Reports (2022)