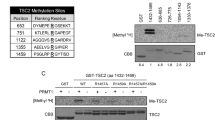
Abstract
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder displaying a large spectrum of symptoms. Linkage studies have shown two loci, TSC1 in 9q34 and TSC2 in 16p13.3, to be involved in the disease. The TSC2 gene, composed of 41 exons, has been isolated and is shown to encode a protein, tuberin, from a 5.5-kb transcript. Mutation screening for both clinical diagnosis and identification of functional domains within the tuberin is in progress. In this study we identify a 33-bp in-frame deletion (1462de133) in the mRNA which segregates in two unrelated French families with severe TSC phenotypes. The corresponding 11 amino acids deletion (aa 482–492) is shown to result from two different splice site mutations at exon 14 and, when compared with the position of two previously described missense mutations, indicates a novel functionally important region of the protein.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Osborne JP, Fryer A, Webb D: Epidemiology of tuberous sclerosis. Ann NY Acad Sci 1991;615:125–127.
Gomez MR: Tuberous Sclerosis, ed 2. New York, Raven Press, 1988.
Gomez MR: Phenotypes of the tuberous sclerosis complex with a revision of diagnostic criteria. Ann NY Acad Sci 1991;615:1–7.
Shepherd CW, Gomez MR, Lie JT, Crowson CS: Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991;66:792–796.
Roach ES, Delgado MR: Tuberous sclerosis. Dermatol Clin 1995;13:151–161.
Roach ES, Smith M, Huttenlocher P, Bat M, Alcorn D, Hawley L: Diagnostic criteria: Tuberous sclerosis complex. J Child Neurol 1992;7:221–224.
Fryer AE, Chalmers A, Connor JM, Fraser I, Povey S, Yates AD, Yates JRW, Osborne JP: Evidence that the gene for tuberous sclerosis is on chromosome 9. Lancet 1987;i:659–661.
Haines JL, Amos J, Attwood J, Bech-Hansen NT, Burley M, Conneally PM, Connor JM, Fashold R, Flodman P, Fryer A, Halley DJJ, Jewell A, Janssen LAJ, Kandt R, Northrup H, Osborne J, Pericak-Vance M, Povey S, Sampson J, Short MP, Smith M, Speer M, Trofatter JA, Yates JRW: Genetic heterogeneity in tuberous sclerosis: study of a large collaborative data set. Ann NY Acad Sci 1991;615:256–264.
Haines JL, Short MP, Kwiatkowsky DJ, Jewell A, Andermann E, Bejjani B, Yang C-H, Gusella JF, Amos JA: Localization of one gene for tuberous sclerosis within 9q32–9q34, and further evidence for heterogeneity. Am J Hum Genet 1991;49:764–772.
Northrup H, Kwiatkowski DJ, Roach ES, Dobyns WB, Lewis RA, Herman GE, Rodriguez E, Daiger SO, Blanton SH: Evidence for genetic heterogeneity in tuberous sclerosis: One locus on chromosome 9 and at least one locus elsewhere. Am J Hum Genet 1992;51:709–720.
Sampson JR, Janssen LAJ, Sandkuijl LA, and the Tuberous Sclerosis Collaborative Group: Linkage investigation of three putative tuberous sclerosis determining loci on chromosome 9q, 11q and 12q. J Med Genet 1992;29:861–866.
Kandt RS, Haines JL, Smith M, Northrup H, Gardner RJ, Short MP, Dumars K, Roach ES, Steingold S, Wall S, Blanton SH, Flodman P, Kwiatkowski DJ, Jewell A, Weber JL, Roses AD, Pericak-Vance MA: Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease. Nat Genet 1992;2:37–41.
Povey S, Burley MW, Attwood J, Benham F, Hunt D, Jeremiah SJ, Franklin D, Gillett G, Malas S, Robson EB, Tippett P, Edwards JH, Kwiatkowski DJ, Super M, Mueller R, Fryer A, Clarke A, Webb D, Osborne J: Two loci for tuberous sclerosis: One on 9q34 and one on 16p13. Ann Hum Genet 1994;58:107–127.
Sampson JR, Harris PC: The molecular genetics of tuberous sclerosis. Hum Mol Genet 1994;3:1477–1480.
Kit-Sing A, Murell J, Buckler A, Blanton SH, Northrup H: Report of a critical recombination further narrowing the TSC1 region. J Med Genet 1996;33:559–561.
European Chromosome 16 Tuberous Sclerosis Consortium: Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305–1315.
Maheshwar MM, Sandford R, Nellist M, Cheadle JP, Sgotto B, Vaudin M, Sampson JR: Comparative analysis and genomic structure of the tuberous sclerosis 2 (TSC2) gene in human and pufferfish. Hum Mol Genet 1996;5:131–137.
Kim KK, Pajak L, Wang H, Field LJ: Cloning, developmental expression, and evidence for alternative splicing of the murine tuberous sclerosis (TSC2) gene product. Cell Mol Biol Res 1995;41:515–526.
Xiao GH, Jin F, Yeung RS: Identification of tuberous sclerosis 2 messenger RNA splice variants that are conserved and differentially expressed in rat and human tissues. Cell Growth Differ 1995;6:1185–1191.
Xu L, Sterner C, Maheshwar MM, Wilson PJ, Nellist M, Short PM, Haines JL, Sampson JR, Ramesh V: Alternative splicing of the tuberous sclerosis 2 (TSC2) gene in human and mouse tissues. Genomics 1995;27:475–480.
Geist RT, Gutmann DH: The tuberous sclerosis 2 gene is expressed at high levels in the cerebellum and developing spinal cord. Cell Growth Differ 1995;6:1305–1315.
Geist RT, Reddly AJ, Zhang J, Gutmann DH: Expression of the tuberous sclerosis 2 gene product, tuberin, in adult and developing nervous system tissues. Neurobiol Dis 1996;3:111–120.
Wienecke R, König A, DeClue JE: Identification of tuberin, the tuberous sclerosis-2 product: Tuberin possesses specific rap1GAP activity. J Biol Chem 1995;270:16409–16414.
Wienecke R, Maize JC Jr, Shoarinejad F, Vass WC, Reed J, Bonifacino JS, Resau JH, de Gunzburg J, Yeung RS, DeClue JE: Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene 1996;13:913–923.
Tsuchiya H, Orimoto K, Kobayashi T, Hino O: Presence of potent transcriptional activation domains in the predisposing tuberous sclerosis 2 (Tsc2) gene product of the Eker rat model. Cancer Res 1996;56:429–433.
Carbonara C, Longa L, Grosso E, Borrone C, Garrè MG, Brisigotti M, Migone N: 9q34 loss of heterozygosity in a tuberous sclerosis astrocytoma suggests a growth suppressor-like activity also for the TSC1 gene. Hum Mol Genet 1994;3:1829–1832.
Green AJ, Smith M, Yates JRW: Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat Genet 1994;6:193–196.
Green AJ, Johnson PH, Yates JRW: The tuberous sclerosis gene on chromosome 9q34 acts as a growth suppressor. Hum Mol Genet 1994;3:1833–1834.
Henske EP, Neuman HPH, Sheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ: Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chrom Cancer 1995;13:295–298.
Carbonara C, Longa L, Grosso E, Mazzucco G, Borrone C, Garrè ML, Brisigotti M, Filippi G, Scabar A, Giannotti A, Falzoni P, Mongua G, Gaini G, Gabrielli M, Riegler P, Danesino C, Ruggieri M, Magro G, Migone N: Apparent preferential loss of heterozygosity at TSC2 over TSC1 chromosomal region in tuberous sclerosis hamartomas. Genes Chrom Cancer 1996; 15:18–25.
Green AJ, Sepp T, Yates JRW: Clonality of tuberous sclerosis hamartomas shown by non-random X-chromosome inactivation. Hum Genet 1996;97:240–243.
Knudson AG: Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971;68:820–823.
Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG: Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 1994;91:11413–11416.
Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O: A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Genet 1995:9:70–74.
Jin F, Wienecke R, Xiao GH, Maize JC Jr, DeClue JE, Yeung RS: Suppression of neoplas-ticigenicity by the wild-type tuberous sclerosis 2 (Tsc2) gene and its C-terminal region. Proc Natl Acad Sci USA 1996;93:9154–9159.
Orimoto K, Tsuchiya H, Kobayashi T, Matsuda T, Hino O: Suppression of the neoplastic phenotype by replacement of the Tsc2 gene in Eker rat renal carcinoma cells. Biochem Biophys Res Commun 1996;219:70–75.
Brook-Carter PT, Peral P, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR: Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease — a contiguous gene syndrome. Nat Genet 1994;8:328–332.
Verhoef S, Vrtel R, van Essen T, Bakker L, Sikkens E, Halley DJJ, Lindhout D, van den Ouweland AMW: Somatic mosaicism and clinical variation in tuberous sclerosis complex. Lancet 1995;345:202.
Kit-Sing A, Rodriguez JA, Rodriguez E Jr., Dobyns WB, Delgado MR, Northrup H: Mutations and polymorphisms in the tuberous sclerosis complex gene on chromosome 16. Hum Mutat 1997;9:23–29.
Kumar A, Wolpert C, Kandt RS, Segal J, Pufky J, Roses AD, Pericak-Vance MA, Gilbert JR: A de novo frame-shift mutation in the tuberin gene. Hum Mol Genet 1995;4:1471–1472.
Kumar A, Kandt RS, Wolpert C, Roses AD, Pericak-Vance MA, Gilbert JR: Mutation analysis of the TSC2 gene in an African-American family. Hum Mol Genet 1995;4:2295–2298.
Vrtel R, Verhoef S, Bouman K, Maheshwar MM, Nellist M, van Essen AJ, Bakker PLG, Hermans CJ, Bink-Boelkens MThE, van Elburg RM, Hoff M, Lindhoust D, Sampson J, Halley DJJ, van den Ouweland AMW: Identification of a nonsense mutation at the 5′ end of the TSC2 gene in a family with a presumptive diagnosis of tuberous sclerosis complex. J Med Genet 1996;33:47–51.
Wilson PJ, Ramesh V, Kristiansen A, Bove C, Jozwiak S, Kwiatkowski DJ, Short MP, Haines JL: Novel mutations detected in the TSC2 gene from both sporadic and familial TSC patients. Hum Mol Genet 1996;5:249–256.
Kumar A, Kandt RS, Wolpert C, Roses AD, Pericak-Vance MA, Gilbert JR: A novel splice site mutation (156+1G -> A) in the TSC2 gene. Hum Mutat 1997;9:64–65.
Jeanpierre M: A rapid method for the purification of DNA from blood. Nucleic Acids Res 1987;15:9611.
Lerman LS, Silverstein K: Computational simulation of DNA melting and its application to denaturing gradient gel eletrophoresis; in Wu R (ed): Methods in Enzymology. New York, Academic Press, 1987, vol 155, pp 482–501.
Myers RM, Maniatis T, Lerman LS: Detection and localization of single base changes by denaturing gradient gel electrophoresis; in Wu R (ed): Methods in Enzymology. New York, Academic Press, 1987, vol 155, pp 501–527.
Fanen P, Ghanem N, Vidaud M, Besmond C, Martin J, Costes B, Plassa F, Goossens M: Molecular characterization of cystic fibrosis: 16 novel mutations identified by analysis of the whole cystic fibrosis conductance transmembrane regulator (CFTR) coding regions and splice site junctions. Genomics 1992; 13:770–776.
Orita M, Suzuki Y, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 1989;5: 874–879.
Shapiro MB, Senapathy P: RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Res 1987; 15: 7155–7174.
Fodde R, Losekoot M: Mutation detection by denaturing gradient gel electrophoresis (DGGE). Hum Mutat 1994;3:83–94.
Krawczak M, Reiss J, Cooper DN: The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet 1992;90:41–54.
Beaudet AL, Tsui LC: A suggested nomenclature for designating mutations. Hum Mutat 1993;2:245–248.
Ad Hoc Committee on Mutation Nomenclature: Update on nomenclature for human gene mutations. Hum Mutat 1996;8:197–202.
Acknowledgements
We would like to thank Sylvie Dumas for helpful discussions and critical reading of the manuscript. We would also like to thank Sylvie Larget and Jean-François Prud’homme from Genethon for their help with cell cultures. This work was supported in part by grants from the Centre National de la Recherche Scientifique (CNRS), the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR), the Association Française contre les Myopathies (AFM), the Association pour la Recherche contre le Cancer (ARC) and Rhône-Poulenc Rorer (RPR). S.J. held a fellowship from the CNRS and the ARC. G.P. held a fellowship from the MENESR and the University Paris 7. We would also like to thank the patients, their families and clinicians for making this work possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jobert, S., Bragado-Nilsson, E., Samolyk, D. et al. Deletion of 11 Amino Acids in Tuberin Associated with Severe Tuberous Sclerosis Phenotypes: Evidence for a New Essential Domain in the First Third of the Protein. Eur J Hum Genet 5, 280–287 (1997). https://doi.org/10.1007/BF03405930
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1007/BF03405930