Abstract
Aim:
To prepare new pharmaceutical forms with sustained delivery properties of recombinant human bone morphogenetic protein-2 (rhBMP2) for tissue engineering and guided tissue regeneration (GTR) use.
Methods:
rhBMP2-loaded dextran-based hydrogel microspheres (rhBMP2-MPs), which aimed to keep rhBMP2 bioactivity and to achieve long-term sustained release of rhBMP2, were prepared by double-phase emulsified condensation polymerization. The physical, chemical performances and biological characteristics of those microspheres were studied both in vitro and in vivo.
Results:
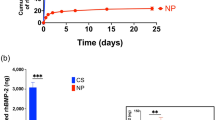
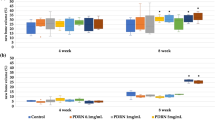
The microspheres' average diameter was 30.33±4.32 μm with 75.4% ranging from 20 μm to 40 μm and the drug loading and encapsulation efficiency were 7.82% and 82.25%, respectively. The rhBMP2-releasing profiles in vitro showed that rhBMP2 release could be maintained more than 10 d. The rhBMP2-MPs, with good swelling and biodegradation behavior, could be kept for 6 months at below 4°C without significant characteristic change or bioactivity loss. Cytology studies showed that rhBMP2-MPs could promote the proliferation of periodontal ligament cells (PDLCs) approximately 10 d, while the bioactivity of concentrated rhBMP2 solution could keep no more than 3 d. Scanning electron microscope showed that rhBMP2-MPs could be enchased into the porous structure of calcium phosphate ceremic (CPC) and the eugonic growth of PDLCs in CPC/rhBMP2-MPs scaffolds. Animal experiments indicated that using CPC/rhBMP2-MPs scaffolds could gain more periodontal tissue regeneration than using rhBMP2 compound firsthand with CPC (CPC/rhBMP2).
Conclusion:
By encapsulating rhBMP2 into dextran-based microspheres, a small quantity of rhBMP2 could achieve equivalent effects to the concentrated rhBMP2 solution and at the same time, could prolong rhBMP2 retention both in vitro and in vivo.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yu TT, Shoichet MS . Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials 2005; 26: 1507–14.
Cheng MH, Brey EM, Allori A, Satterfield WC, Chang DW, Patrick CW Jr, et al. Ovine model for engineering bone segments. Tissue Eng 2005; 11: 214–25.
Choo AB, Padmanabhan J, Chin AC, Oh SK . Expansion of pluri-potent human embryonic stem cells on human feeders. Biotechnol Bioeng 2004; 88: 321–31.
Hsu SH, Tsai CL, Tang CM . Evaluation of cellular affinity and compatibility to biodegradable polyesters and type-II collagen-modified scaffolds using immortalized rat chondrocytes. Artif Organs 2002; 26: 647–58.
Kim SS, Vacanti JP . The current status of tissue engineering as potential therapy. Semin Pediatr Surg 1999; 8: 119–23.
Sorensen RG, Wikesjo UM, Kinoshita A, Wozney JM . Periodontal repair in dogs: evaluation of a bioresorbable calcium phosphate cement (Ceredex) as a carrier for rhBMP-2. J Clin Periodontol 2004; 31: 796–804.
Wikesjo UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM . Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol 2004; 31: 662–70.
Hanisch O, Sorensen RG, Kinoshita A, Spiekermann H, Wozney JM, Wikesjo UM . Effect of recombinant human bone morphogenetic protein-2 in dehiscence defects with non-submerged immediate implants: an experimental study in Cynomolgus monkeys. J Periodontol 2003; 74: 648–57.
Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB . A reentry study on the use of bovine porous bone mineral, GTR, and platelet-rich plasma in the regenerative treatment of intrabony defects in humans. Int J Periodontics Restorative Dent 2005; 25: 49–59.
Wikesjo UM, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res 2004; 15: 194–204.
Wikesjo UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR . Periodontal repair in dogs: space-providing ePTFE devices increase rhBMP-2/ACS-induced bone formation. J Clin Periodontol 2003; 30: 715–25.
Wikesjo UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR . Periodontal repair in dogs: rhBMP-2 significantly enhances bone formation under provisions for guided tissue regeneration. J Clin Periodontol 2003; 30: 705–14.
Ripamonti U, Reddi AH . Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med 1997; 8: 154–63.
Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE . Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol 2003; 74: 1282–92.
Park YJ, Ku Y, Chung CP, Lee SJ . Control release of platelet-derived growth factor from porous poly (L-lactide) membranes for guided tissue regeneration. J Control Release 1998 12; 51: 201–11.
Wikesjo UM, Razi SS, Sigurdsson TJ, Tatakis DN, Lee MB, Ongpipattanakul B, et al. Periodontal repair in dogs: effect of recombinant human transforming growth factor-beta 1 on guided tissue regeneration. J Clin Periodontol 1998; 25: 475–81.
Rimondini L, Nicoli-Aldini N, Fini M, Guzzardella G, Tschon M, Giardino R . In vivo experimental study on bone regeneration in critical bone defects using an injectable biodegradable PLA/PGA copolymer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: 148–54.
Hench LL, Xynos ID, Polak JM . Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed 2004; 15: 543–62.
Schmokel HG, Weber FE, Seller G, von Rechenberg B, Schense JC, Schawalder P, et al. Treatment of nonunions with nonglycosylated recombinant human bone morphogenetic protein-2 delivered from a fibrin matrix. Vet Surg 2004; 33: 112–8.
Holland TA, Tessmar JK, Tabata Y, Mikos AG . Transforming growth factor-beta 1 release from oligo (poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release 2004; 94: 101–14.
Vandelli MA, Rivasi F, Guerra P, Forni F, Arletti R . Gelatin microspheres crosslinked with D,L-glyceraldehyde as a potential drug delivery system: preparation, characterisation, in vitro and in vivo studies. Int J Pharm 2001; 215: 175–84.
Brime B, Ballesteros MP, Frutos P . Preparation and in vitro characterization of gelatin microspheres containing levodopa for nasal administration. J Microencapsul 2000; 17: 777–84.
Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, et al. Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharmacol Sci 2001; 13: 179–85.
Nakase H, Okazaki K, Tabata Y, Uose S, Ghana M, Uchida K, et al. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J Pharmacol Exp Ther 2000; 292: 15–21.
Chen FM, Wu ZF, Jin Y, Wang QT, Wu H, Wang GF, et al. Development of a hydrogel microsphere delivery system for rhBMP2. J Pract Stomatol 2005; 21: 174–7 Chinese.
Someman MJ, Foster RA, Vorsteg GM, Progebin K, Wynn RL . Effects of minocycline on fibroblast attachment and spreading. J Periodontal Res 1988; 23: 154–9.
Coletta RD, Almeida OP, Graner E, Page RC, Bozzo L . Differential proliferation of fibroblast cultured from hereditary gingival fibromatosis and normal gingival. J Periodontal Res 1998; 33: 469–75.
Lindhe J, Pontoriero R, Berglundh T, Araujo M . The effect of flap management and bioresorbable occlusive devices in GTR treatment of degree III furcation defects. An experimental study in dogs. J Clin Periodontol 1995; 22: 276–83.
Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS . Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 2002; 249: 165–74.
Stenekes RJ, Franssen O, van Bommel EM, Crommelin DJ, Hennink WE . The preparation of dextran microspheres in an all-aqueous system: effect of the formulation parameters on particle characteristics. Pharm Res 1998; 15: 557–61.
Kamath KR, Park K . Biodegradable hydrogels in drug delivery. Adv Drug Delivery Rev 1993; 11: 59–84.
Kuijpers AJ, van Wachem PB, van Luyn MJ, Brouwer LA, Engbers GH, Krijgsveld J, et al. In vitro and in vivo evaluation of gelatin-chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials 2000; 21: 1763–72.
Chourasia MK, Jain SK . Polysaccharides for colon targeted drug delivery. Drug Deliv 2004; 11: 129–48.
Harada M, Murata JI, Sakamura Y, Sakakibara H, Okuno S, Suzuki T . Carrier and dose effects on the pharmacokinetics of T-0128, a camptothecin analogue-carboxymethyl dextran conjugate, in non-tumor- and tumor-bearing rats. J Control Release 2001; 71: 71–86.
Kojima T, Hashida M, Muranishi S, Sezaki HJ . Mitomyc in C dextran conjugate: a novel high molecular weight pro-drug of mitomycin C. J Pharm Pharmacol 1980; 32: 30–4.
Williams AS, Taylor G . Synthesis, characterization and release of cromoglycate from dextran conjugates. Int J Pharm 1992; 83: 233–9.
Kim IS, Jeong YI, Kim DH, Lee YH, Kim SH . Albumin release from biodegradable hydrogels composed of dextran and poly (ethylene glycol) macromer. Arch Pharm Res 2001; 24: 69–73.
Sery TW, Herhe EJ . Degradation of dextrans by enzymes of intestinal bacteria. J Bacteriol 1956; 71: 373–80.
Brondsted H, Andersen C, Hovgaard L . Crosslinked dextran—a new capsule material for colon targeting of drugs. J Control Release 1998; 53: 7–13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by Hi-Tech Research and Development Program (863 Program) of China (No 2002AA205041).
Rights and permissions
About this article
Cite this article
Chen, Fm., Wu, Zf., Wang, Qt. et al. Preparation of recombinant human bone morphogenetic protein-2 loaded dextran-based microspheres and their characteristics. Acta Pharmacol Sin 26, 1093–1103 (2005). https://doi.org/10.1111/j.1745-7254.2005.00180.x
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1111/j.1745-7254.2005.00180.x
Keywords
This article is cited by
-
Composite glycidyl methacrylated dextran (Dex-GMA)/gelatin nanoparticles for localized protein delivery
Acta Pharmacologica Sinica (2009)
-
Bioactivation of knitted cellulose scaffolds by strontium
Cellulose (2008)
-
Preparation of BMP-2 Containing Bovine Serum Albumin (BSA) Nanoparticles Stabilized by Polymer Coating
Pharmaceutical Research (2008)