Abstract
Aim:
The resurgence of severe acute respiratory syndrome (SARS) is still a threat because the causative agent remaining in animal reservoirs is not fully understood, and sporadic cases continue to be reported. Developing high titers of anti-SARS hyperimmune globulin to provide an alternative pathway for emergent future prevention and treatment of SARS.
Methods:
SARS coronavirus (CoV)F69 (AY313906) and Z2-Y3 (AY394989) were isolated and identified from 2 different Cantonese onset SARS patients. Immunogen was prepared from SARS-CoV F69 strain. Six health horses were immunized 4 times and serum was collected periodically to measure the profile of specific IgG and neutralizing antibodies using indirect enzyme-linked immunosorbent assay and a microneutralization test. Sera were collected in large amounts at the peak, where IgG was precipitated using ammonium sulphate and subsequently digested with pepsin. The product was then purified using anion-exchange chromatography to obtain F(ab′)2 fragments.
Results:
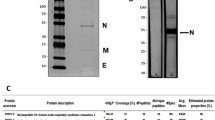
The specific IgG and neutralizing antibody titers peaked at approximately week 7 after the first immunization, with a maximum value of 1:14210. The sera collected at the peak were then purified. Fragment of approximately 15 g F(ab′)2 was obtained from 1 litre antiserum and the purity was above 90% with the titer of 1:5120, which could neutralize the other strain (SARS-CoV Z2-Y3) as well.
Conclusion:
This research provides a viable strategy for the prevention and treatment of SARS coronavirus infection with equine hyperimmune globulin, with the purpose of combating any resurgence of SARS.
Similar content being viewed by others
Article PDF
References
Drosten C, Gunther S, Preiser, W, van der Werf, S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–76.
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953–66.
Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle, JP, et al Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 2003; 300: 1394–9.
World Health Organization [homepage on the Internet]. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Geneva: The Organization. [cited 2005 Jul 9] Available from: http://www.who.int/csr/sars/country/table2004_04_21/en/.
Lim PL, Asok K, Gopalakrishna G, Chan KP, Wong CW, et al. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med 2004; 250: 1740–5.
World Health Organization [homepage on the Internet]. Severe acute respiratory syndrome (SARS) in Singapore. Geneva: The Organization. [ cited 2005 April 10] Available from: http://www.who.int/csr/don/2003_09_10/en/.
World Health Organization [homepage on the Internet]. Severe Acute Respiratory Syndrome (SARS) in Taiwan, China. Geneva: The Organization. [ cited 2005 May 17] Available from: http://www.who.int/csr/don/2003_12_17/en/.
World Health Organization [homepage on the Internet]. SARS: one suspected case reported in China. Geneva: The Organization. [ cited 2005 April 22] Available from: http://www.who.int/csr/don/2004_04_22/en/.
World Health Organization [homepage on the Internet]. China's latest SARS outbreak has been contained, but biosafety concerns remain-Update 7. Geneva: The Organization. [ cited 2005 July 18] Available from: http://www.who.int/csr/don/2004_05_18a/en/.
Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, et al. Newly discovered coronavirus as the primary cause of Severe Acute Respiratory Syndrom. Lancet 2003; 362: 263–70.
Holmes KV . SARS-Associated Coronavirus. N Engl J Med 2003; 348: 1948–51.
Saif LJ, Bohl EH . Passive immunity to transmissible gastroenteritis virus: intramammary viral inoculation of sows. Ann N Y Acad Sci 1983; 409: 708–23.
Sestak K, Lanza I, Park SK, Weilnau PA, Saif LJ . Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am J Vet Res 1996; 57: 664–71.
Homberger FR, Barthold SW . Passively acquired challenge immunity to enterotropic coronavirus in mice. Arch Virol 1992; 126: 35–43.
Arthington, JD, Jaynes CA, Tyler HD, Kapil S, Quigley, JD 3rd . The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves1. J Dairy Sci 2002; 85: 1249–54.
Li G, Chen X, Xu A . Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med 2003; 349: 508–9.
Wong VW, Dai D, Wu AK, Sung JJ . Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J 2003; 9: 199–201.
Wilde H, Chomchey P, Punyaratabandhu P, Phanupak P, Chutivongse S . Purified equine rabies immune globulin: a safe and affordable alternative to human rabies immune globulin. Bull World Health Organ 1989; 67: 731–6.
Wilde H, Chutivongse S . Equine rabies immune globulin: a product with an undeserved poor reputation. Am J Trop Med Hyg 1990; 42: 175–8.
Yan XG, Wan ZY, Zhang X, Chen QX, ZHeng K, Huang JC, et al. Isolation and identification of SARS coronavirus in Guangdong province. Chin J Exp Clin Virol 2003; 17: 213–6.
Lu, JH, Yan XG, GUO ZM, ZHeng HY, Zhang X, Wan ZY, et al. Establishment of SARS virus vaccine line (F69), Guangdong Med J 2003; 24 ( SARS Suppl II): 225–7.
Lu, JH, ZHeng HY, Yan XG, Wan ZY, Zhang RL, Meng JX, et al. Establishment of SARS virus vaccine line (Y3), Guangdong Med J 2003; 24 ( SARS Suppl II): 234–6.
Laemmli UK . Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–5.
Sampathkumar P, Temesgen Z, Smith TF, Thompson RL . SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin Proc 2003; 78: 882–90.
Quiambao BP, Lang J, Vital S, Montalban CG, Le Mener, V, Wood SC, et al. Immunogenicity and effectiveness of post–exposure rabies prophylaxis with a new chromatographically purified Verocell rabies vaccine (CPRV): a two-stage randomised clinical trial in the Philippines. Acta Trop 2000; 75: 39–52.
Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA 2004; 101: 2536–41.
Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 2004; 303: 1666–9.
Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis 1999; 179 Suppl 1: 224–34.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the LIC Foundation of Hong Kong and the Science Foundation of Guangdong Province (No 2003Z3-E0461).
Rights and permissions
About this article
Cite this article
Lu, Jh., Guo, Zm., Han, Wy. et al. Preparation and development of equine hyperimmune globulin F(ab′)2 against severe acute respiratory syndrome coronavirus. Acta Pharmacol Sin 26, 1479–1484 (2005). https://doi.org/10.1111/j.1745-7254.2005.00210.x
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1111/j.1745-7254.2005.00210.x