Abstract
Aim:
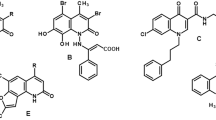
To design and synthesize a novel class of protein tyrosine kinase inhibitors, featuring the N-(2-oxo-1,2-dihydroquinolin-3-yl-methyl)-thiourea framework.
Methods:
First, compounds 1 and 2 were identified using the virtual screening approach in conjunction with binding assay based on surface plasmon resonance. Subsequently, 3 regions of compounds 1 and 2 were selected for chemical modification. All compounds were characterized potent inhibitory activities toward the human lung adenocarcinoma cell line SPAC1.
Results:
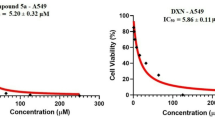
Forty new compounds (1–2, 3a–g, 4a–w, and 5a–l) were designed, synthesized and bioassayed. Six compounds (1, 3e, 4l, 4w, 5a, and 5b) were found to show promising inhibitory activity against the SPAC1 tumor cell line. The inhibitory activity of compound 5a increases approximately 10 times more than that of the original compound 1.
Conclusion:
This study provides a promising new template with potential antitumor activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aaronson SA . Growth factors and cancer. Science 1991; 254: 1146–52.
Bridges AJ . Chemical inhibitors of protein kinases. Chem Rev 2001; 101: 2541–72.
Ullrich A, Schlessinger J . Signal transduction by receptors with tyrosine kinase activity. Cell 1990; 61: 203–12.
Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, et al. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science 1989; 243: 811–4.
Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P . Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta 2001; 1550: 144–52.
Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, et al. Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev 2001; 21: 499–512.
Traxler P, Bold G, Frei J, Lang M, Lydon N, Mett H, et al. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J Med Chem 1997; 40: 3601–16.
Khazaie K, Shirrmacher V, Lichtner RB . EGF receptor in neoplasia and metastasis. Cancer Metast Rev 1993; 12: 155–274.
Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 1994; 265: 1093–5.
Chinkers M, Brugge JS . Characterization of structural domains of the human epidermal growth factor receptor obtained by partial proteolysis. J Biol Chem 1984; 259: 11 534–42.
Carpenter CD, Ingraham HA, Cochet C, Walton GM, Lazar CS, Sowadski JM, et al. Structural analysis of the transmembrane domain of the epidermal growth factor receptor. J Biol Chem 1991; 266: 5750–5.
Araujo RP, Petricoin EF, Liotta LA . A mathematical model of combination therapy using the EGFR signaling network. Biosystems 2005; 80: 57–9.
Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, et al. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2). J Med Chem 2003; 46: 49–63.
Tsou HR, Mamuya N, Johnson BD, Reich MF, Gruber BC, Ye F, et al. 6-Substituted-4-(3-bromophenylamino) quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity. J Med Chem 2001; 44: 2719–34.
Mu F, Coffing SL, Riese DJ, Geahlen RL, Verdier-Pinard P, Hamel TE, et al. Design, synthesis, and biological evaluation of a series of lavendustin A analogues that inhibit EGFR and Syk tyrosine kinases, as well as tubulin polymerization. J Med Chem 2001; 44: 441–52.
Jin Y, Li HY, Lin LP, Tan J, Ding J, Luo X, et al. Synthesis and antitumor evaluation of novel 5-substituted-4-hydroxy-8-nitroquinazolines as EGFR signaling-targeted inhibitors. Bioorg Med Chem 2005; 13: 5613–22.
Albuschat R, Löwe W, Weber M, Luger P, Jendrossek V . 4-Anilinoquinazolines with lavendustin A subunit as inhibitors of epidermal growth factor receptor tyrosine kinase: syntheses, chemical and pharmacological properties. Eur J Med Chem 2004; 39: 1001–11.
Asano T, Yoshikawa T, Usui T, Yamamoto H, Yamamoto Y, Uehara Y, et al. Benzamides and benzamidines as specific inhibitors of epidermal growth factor receptor and v-Src protein tyrosine kinases. Bioorg Med Chem 2004; 12: 3529–42.
Supuran CT, Scozzafava A . Protein tyrosine kinase inhibitors as anticancer agents. Expert Opin Ther Patents 2004; 14: 35–53.
Chen G, Luo X, Zhu W, Luo C, Liu H, Puah CM, et al. Elucidating inhibitory models of the inhibitors of epidermal growth factor receptor by docking and 3D-QSAR. Bioorg Med Chem 2004; 12: 2409–17.
Norman P . ZD-1839 (astraZeneca). Curr Opin Investig Drugs 2001; 3: 428–34.
Norman P . OSI-774 OSI pharmaceuticals. Curr Opin Investig Drugs 2001; 2: 298–304.
Smaill JB, Palmer BD, Rewcastle GW, Denny WA, McNamara DJ, Dobrusin EM, et al. Tyrosine kinase inhibitors. 15. 4-(phenylamino)quinazoline and 4-(phenylamino)pyrido[d]pyrimidine acrylamides as irreversible inhibitors of the ATP binding site of the epidermal growth factor receptor. J Med Chem 1999; 42: 1803–15.
Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA 1998; 95: 12022–7.
Wissner A, Johnson BD, Floyd MB, Kitchen DB, inventors; American Cyanamid Company, assignee. 4-Aminoquinazolines as EGFR inhibitors. US Patent 5 760 041. 1998; Jun 2.
Lee MW, Seo CW, Kim SW, Yang HJ, Lee HW, Choi JH, et al. Cutaneous side effects in non-small cell lung cancer patients treated with Iressa (ZD1839), an inhibitor of epidermal growth factor. Acta Derm Venereol 2004; 84: 23–6.
Kuntz ID, Meng EC, Shoichet BK . Receptor-based molecular design. Acc Chem Res 1994; 27: 117–23.
Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 2000; 97: 7124–9.
Liu H, Li Y, Song M, Tan X, Cheng F, Zheng S, et al. Structure-based discovery of potassium channel blockers from natural products: Virtual screening and electrophysiological assay testing. Chem Biol 2003; 10: 1103–13.
Stamos J, Sliwkowski MX, Eigenbrot C . Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002; 277: 46 265–72.
Li J, Chen J, Gui C, Zhang L, Qin Y, Xu Q, et al. Discovering novel chemical inhibitors of human cyclophilin A: virtual screening, synthesis, and bioassay. Bioorg Med Chem 2006; 14: 2209–24.
Ewing T, Kuntz ID . Critical evaluation of search algorithms used in automated molecular docking. J Comput Chem 1997; 18: 1175–89.
Kuntz ID . Structure-based strategies for drug design and discovery. Science 1992; 257: 1078–82.
Weiner SJ, Kollman PA, Nguyen DT, Case DA . An all atom force field for simulations of proteins and nucleic acids. J Comput Chem 1986; 7: 230–52.
Gasteiger J, Marsili M . Iterative partial equalization of orbital electronegativity-A rapid access to atomic charges. Tetrahedron 1980; 36: 3219–28.
Muegge I, Rarey M . Small molecule docking and scoring. In: Lipkowitz KB, Boyd, DB Reviews in computational chemistry. New York: John Wiley & Sons; 2001. p 1–60.
Charifson PS, Corkery JJ, Murcko MA, Walters WP . Consensus scoring: a method for obtaining improved hit rates from docking databases of three-dimensional structures into proteins. J Med Chem 1999; 42: 5100–9.
Sybyl molecular modeling package (Computer program). Version 6.8. St Louis, MO: Tripos Associates, 2000.
Traxler P, Furet P, Mett H, Buchdunger E, Meyer T, Lydon N . Design and synthesis of novel tyrosine kinase inhibitors using a pharmacophore model of the ATP-binding site of the EGF-R. J Pharm Belg 1997; 52: 88–96.
Meth-Cohn O, Narine B, Tarnowski B . A versatile new synthesis of quinolines and related fused pyridines. Part 5. The synthesis of 2-chloroquinoline-3-carbaldehydes. J Chem Soc Perkin Trans 1 1981; 5: 1520–30.
Meth-Cohn O, Narine B, Tarnowski B, Hayes R, Keyzad A, Rhouati S, et al. A versatile new synthesis of quinolines and related fused pyridines. Part 9. Synthetic application of the 2-chloroquinoline-3-carbaldehydes. J Chem Soc Perkin Trans 1 1981; 9: 2509–17.
Tilakraj T, Ambekar SY . Synthesis and mass spectra of some 2H-pyrano[2,3-b]quinolin-2-ones. J Indian Chem Soc 1985; 62: 251–3.
Guo XN, Zhong L, Zhang XH, Zhao WM, Zhang XW, Lin LP, et al. Evaluation of active recombinant catalytic domain of human ErbB-2 tyrosine kinase, and suppression of activity by a naturally derived inhibitor, ZH-4B. Biochim Biophys Acta 2004; 1673: 186–93.
Sargent JM, Taylor CG . Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer 1989; 60: 206–10.
Rewcastle GW, Denny WA, Bridges AJ, Zhou H, Cody DR, McMichael A, et al. Tyrosine kinase inhibitors. 5. Synthesis and structure-activity relationships for 4-[(phenylmethyl)amino]-and 4-(phenylamino)quinazolines as potent adenosine 5′-triph-osphate binding site inhibitors of the tyrosine kinase domain of the epidermal growth factor receptor. J Med Chem 1995; 38: 3482–7.
Author information
Authors and Affiliations
Additional information
Project supported by the State Key Program of Basic Research of China (No 2002CB512802), the 863 Hi-Tech Program (No 2002AA233061, 2002AA104270) and the National Natural Science Foundation of China (No 20372069, 29725203, and 20472094).
Rights and permissions
About this article
Cite this article
Li, J., Tan, Jz., Chen, Ll. et al. Design, synthesis and antitumor evaluation of a new series of N-substituted-thiourea derivatives. Acta Pharmacol Sin 27, 1259–1271 (2006). https://doi.org/10.1111/j.1745-7254.2006.00437.x
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1111/j.1745-7254.2006.00437.x
Keywords
This article is cited by
-
A novel catalyst-free method for the synthesis of mono-N-substituted thioureas in water
Journal of the Iranian Chemical Society (2024)
-
Antimicrobial and anticancer activity of some novel fluorinated thiourea derivatives carrying sulfonamide moieties: synthesis, biological evaluation and molecular docking
Chemistry Central Journal (2017)
-
Microwave-assisted CAN-catalyzed solvent-free synthesis of N-allyl quinolone-based pyrano[4,3-b]chromene and benzopyrano[3,2-c]chromene derivatives and their antimicrobial activity
Medicinal Chemistry Research (2013)
-
Synthesis and in vitro antimicrobial evaluation of novel 2-amino-6-(phenylthio)-4-(2-(phenylthio)quinolin-3-yl)pyridine-3,5-dicarbonitriles
Medicinal Chemistry Research (2013)
-
Chemical space sampling by different scoring functions and crystal structures
Journal of Computer-Aided Molecular Design (2010)