Abstract
Aim:
The relative bioavailabilities and effects on lung injury alleviation of 4 insulin-artificial pulmonary surfactant (INS-APS) preparations were studied in normal rats. The relationship between the minimal surface tension (γmin) of INS-APS and the absorption of insulin was also investigated.
Methods:
Four formulations of APS [1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)/lecithin/palmitic acid (PA), DPPC/1-hexadecanol (Hex)/tyloxapol (Tyl), DPPC/L-α-phosphatidyl-DL-glycerol sodium salt (PG), DPPC/Tyl] were prepared by thin-film sonication method and direct sonication. The γmin of 4 APS dispersions was examined with and without INS by pulsating with a bubble surface tensiometer. In vivo experiments were performed in which serum glucose change and the insulin level were measured by an enzymatic glucose reagent kit and a radioimmunology assay kit after IT to rats. The reduction in lung injury by INS-APS following 7 d of consecutive administration was evaluated by the pulmonary edema index (the weight ratio of wet lung to dry lung) and histopathology examination.
Results:
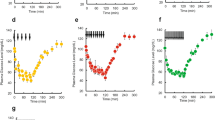
The γmin of all APS dispersions were below 10 mN/m. There was no significant difference (P> 0.05) between the γmin of APS and the corresponding INS-APS. In vivo experiments showed a significant glucose level decrease and insulin absorption increase (P<0.05) in the presence of APS, compared to the insulin solution alone. From the results, we found that the pulmonary edema index values of all the INS-APS groups were significant lower (P<0.05) than that of the insulin solution group, and there were no significant differences (P>0.05) between INS/DPPC/Tyl, INS/DPPC/PG, and the control group. The pulmonary edema indices and histopathological observation indicated that INS-APS could alleviate lung injury.
Conclusion:
The most potent hypoglycemic effect and insulin absorption increase in this study were obtained with INS/DPPC/Tyl. According to the results, there was a linear correlation between the γmin and relative bioavailability of INS-APS, suggesting a possible effect of the γmin of carriers on the in vivo absorption of insulin. APS, DPPC/Tyl, and DPPC/PG dispersions might be the most efficient insulin pulmonary delivery carriers in achieving a lower γmin, enhancing insulin absorption, and decreasing lung injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N . The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res 2001; 2: 198–209.
Merrill JD, Ballard RA . Pulmonary surfactant for neonatal respiratory disorders. Curr Opin Pediatr 2003; 15: 149–54.
Erpenbeck VJ, Hagenberg A, Dulkys Y, Elsner D, Balder R, Krentel H, et al. Natural porcine surfactant augments airway inflammation after allergen challenge in patients with asthma. Am J Respir Crit Care Med 2004; 169: 578–86.
Van't Veen A, Gommers D, Mouton JW, Kluytmans JA, Krijt EJ, Lachmann B . Exogenous pulmonary surfactant as a drug delivering agent: influence of antibiotics on surfactant activity. Br J Pharmacol 1996; 118: 593–8.
Katkin JP, Husser RC, Langston C, Welty SE . Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum Gene Ther 1997; 8: 171–6.
Jiang ZQ, Sun GM, Ma YM . Artificial reconstituted pulmonary surfactant in prevention and treatment of respiratory distress syndrome in neonates. Acta Pharmacol Sin 1997; 18: 182–4.
Mitra R, Pezron I, Li Y, Mitra AK . Enhanced pulmonary delivery of insulin by lung lavage fluid and phospholipids. Int J Pharm 2001; 217: 25–31.
Enhorning G . Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol 1977; 43: 198–203.
Ji Y, Pei YY . Primary study of artificial pulmonary surfactant as carrier of insulin pulmonary delivery. Chin Pharm J 2006; 41: 766–8.
Paddy JF . Surface tension. Part III. Tables relating the size and shape of liquid drops to the surface tension. In Matijevic E, editor. Surface and colloid science. New York: Wiley-Interscience; 1969. p 151–97.
Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm 2002; 249: 139–47.
Lu J, Ji Y, Jiang ZQ . Study on the bioavailability and initial investigation on the rat following pulmonary delivery of insulin lipid suspension. Chin J Biochem Pharm 2002; 23: 271–4.
Zhang Q, Shen ZC, Tsuneji N . Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm 2001; 218: 75–80.
Kharasch VS, Sweeney TD, Fredberg J, Lehr J, Damokosh AI, Avery ME, et al. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am Rev Respir Dis 1991; 144: 909–13.
Enhorning G . Pulmonary surfactant function studied with the pulsating bubble surfactometer (PBS) and the capillary surfactometer (CS). Comp Biochem Physiol A Mol Integr Physiol 2001; 129: 221–6.
Tanaka Y, Takei T, Aiba T, Masuda K, Kiuchi A, Fujiwara T . Development of synthetic lung surfactants. J Lipid Res 1986; 27: 475–85.
Latallo JFK, Chen CL, Eichman J, Bielinska AU, Baker JR . Enhancement of endrimer–mediated transfection using synthetic lung surfactant exosurf neonatal in vitro. Biochem Biophys Res Commun 1999; 264: 253–61.
Lewis JF, Brackenbury A . Role of exogenous surfactant in acute lung damage. Crit Care Med 2003; 31: S324–8.
Palmblad M, Gustafsson M, Curstedt T, Johansson J, Schurch S . Surface activity and film formation from the surface associated material of artificial surfactant preparations. Biochim Biophys Acta 2001; 1510: 106–17.
Park SY, Hannemann RE, Franses EI . Dynamic tension and adsorption behavior of aqueous lung surfactants. Colloids Surf B Biointerfaces 1999; 15: 325–38.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Ministry of Education Grant of China (2001) and a grant from the Department of Public Health of Shanghai (2001).
Rights and permissions
About this article
Cite this article
Ji, Y., Liu, C. & Pei, Yy. Artificial pulmonary surfactant as a carrier for intratracheally instilled insulin. Acta Pharmacol Sin 28, 744–750 (2007). https://doi.org/10.1111/j.1745-7254.2007.00513.x
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1111/j.1745-7254.2007.00513.x