Abstract
Aim:
Insulin-like growth factor-1 (IGF-1) is an important hypertrophic and cell cycle progression factor for a number of cell types. It has been proven that IGF-1 is involved in the regulation of thyroid proliferation and cell cycle progression; however, the exact mechanism of this regulation has not been fully elucidated. In the present study, we investigated the effect of IGF-1 on the expression of cyclin D1, an important cell cycle regulatory protein, and a signaling pathway involved in IGF-1's effect on cyclinD1 expression in FRTL thyroid cells.
Methods:
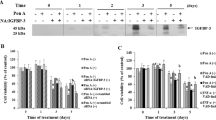
FRTL thyroid cells were treated with IGF-1 or vector control for 24 h. As appropriate to individual experiments, a phosphatidylinositol 3-kinase (PI3K) inhibitor, LY294002, and/or a nuclear factor-κB (NF-κB) inhibitor, BAY11-7082, were added 1 h prior to IGF-1 treatment. Western blotting was used to detect cyclin D1 protein expression. Immunofluorescence was performed to analyze the expression of IκBα, an NF-κB inhibitory protein. Cell cycle analysis was performed by fluorescence activated cell sorting (FACS).
Results:
IGF-1 increased the cyclin D1 expression in thyroid cells. This increase was blocked by pretreatment with LY294002 or BAY11-7082. Further studies showed that IGF-1 specifically induced NF-κB activity. Treatment with IGF-1 could accelerate cell cycle progression from G0/G1 to S phase, whereas this progression was inhibited by the presence of LY294002 or BAY11-7082.
Conclusion:
In summary, the results of the present study show that in FRTL cells, IGF-1 promotes cell cycle progression via an upregulation of cyclin D1 expression, at least partially through the PI3K/NF-κB signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Graña X, Reddy EP . Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995; 11: 211–9.
Sherr CJ, Kato J, Quelle DE, Matsuoka M, Roussel MF. D-type cyclins and their cyclin-dependent kinases: G1 phase integrators of the mitogenic response. Cold Spring Harb Symp Quant Biol 1994; 59: 11–9.
Lantsov D, Meirmanov S, Nakashima M, Kondo H, Saenko V, Naruke Y, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant β–catenin expression. Histopathology 2005; 47: 248–56.
Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, et al. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells 2004; 22: 1239–45.
Radcliff K, Tang TB, Lim J, Zhang Z, Abedin M, Demer LL, et al. Insulin-like growth factor-1 regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res 2005; 96: 398–400.
Ghosh S, May MJ, Kopp EB. NF-kappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16: 225–60.
Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 1999; 18: 6910–24.
Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-kappaB activity. Annu Rev Immunol 2000; 18: 621–63.
Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest 2001; 107: 7–11.
Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18: 6853–66.
Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol 1999; 19: 2690–8.
Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 1999; 19: 5785–99.
Bertrand F, Philippe C, Antoine PJ, Baud L, Groyer A, Capeau J, et al. Insulin activates nuclear factor kappaB in mammalian cells through a Raf-1-mediated pathway. J Biol Chem 1995; 270: 24435–41.
Heck S, Lezoualc'h F, Engert S, Behl C . Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J Biol Chem 1999; 274: 9828–35.
Xiong W, Pestell RG, Watanabe G, Li J, Rosner MR, Hershenson MB . Cyclin D1 is required for S phase traversal in bovine tracheal myocytes. Am J Physiol 1997; 16: L1205–10.
Beier F, Lee RJ, Taylor AC, Pestell RG, LuValle P . Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc Natl Acad Sci USA 1999; 96: 1433–8.
Jiang Y, Cheng DW, Levi E, Singh LP. IGF-1 increases laminin, cyclin D1, and P21Cip1 expression in glomerular mesangial cells: An investigation of the intracellular signaling pathway and cell-cycle progression. J Cell Biochem 2006; 98: 208–20.
Wilker E, Lu J, Rho O, Carbajal S, Beltrán L, DiGiovanni J . Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinog 2005; 44: 137–45.
Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle 2004; 3: 372–9.
Zaka M, Rafi MA, Rao HZ, Luzi P, Wenger DA. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Mol Cell Neurosci 2005; 30: 398–407.
Saito J, Kohn AD, Roth RA, Noguchi Y, Tatsumo I, Hirai A, et al. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid 2001; 11: 339–51.
Segev DL, Umbricht C, Zeiger MA. Molecular pathogenesis of thyroid cancer. Surg Oncol 2003; 12: 69–90.
Baldwin AS Jr, Azizkhan JC, Jensen DE, Beg AA, Coodly LR. Induction of NF-kappaB DNA-binding activity during the G0-to-G1 transition in mouse fibroblasts. Mol Cell Biol 1991; 11: 4943–51.
Duckett CS, Perkins ND, Leung K, Agranoff AB, Nabel GJ . Cytokine induction of nuclear factor kappa B in cycling and growth-arrested cells. Evidence for cell cycle-independent activation. J Biol Chem 1995; 270: 18836–40.
Corbetta S, Vicentini L, Ferrero S, Lania A, Mantovani G, Cordella D, et al. Activity and function of the nuclear factor kappaB pathway in human parathyroid tumors. Endocr Relat Cancer 2005; 12: 929–37.
Aksoy E, Vanden Berghe W, Detienne S, Amraoui Z, Fitzgerald KA, Haegeman G, et al. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-kappa B activation and IFN-β synthesis downstream of Toll-like receptor 3 and 4. Eur J Immunol 2005; 35: 2200–9.
Jimenez Del Rio M, Velez-Pardo C. Insulin-like growth factor-1 prevents Abeta[25–35]/(H2O2)- induced apoptosis in lymphocytes by reciprocal NF-kappaB activation and p53 inhibition via PI3K-dependent pathway. Growth Factors 2006; 24: 67–78.
Acknowledgements
Project supported by the National Natural Science Foundation of China (30672748) and Shandong Administration of Traditional Chinese Medicine (2005-070).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, M., Zhong, X., Ma, Cy. et al. Insulin-like growth factor-1 promotes cell cycle progression via upregulation of cyclin D1 expression through the phosphatidylinositol 3-kinase/nuclear factor-κB signaling pathway in FRTL thyroid cells. Acta Pharmacol Sin 30, 113–119 (2009). https://doi.org/10.1038/aps.2008.8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2008.8
Keywords
This article is cited by
-
Biotherapeutic Applications of Platelet-Rich Plasma in Regenerative Medicine
Tissue Engineering and Regenerative Medicine (2023)
-
Low-temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-activated NF-κB Signaling Pathway
Current Medical Science (2018)
-
HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2
Tumor Biology (2016)
-
Early treatment with xenon protects against the cold ischemia associated with chronic allograft nephropathy in rats
Kidney International (2014)
-
Diosgenin relieves goiter via the inhibition of thyrocyte proliferation in a mouse model of Graves' disease
Acta Pharmacologica Sinica (2014)