Abstract
Aim:
KCNQ4 channels play an important part in adjusting the function of cochlear outer hair cells. The aim of this study was to investigate the effects of ser/thr phosphatase inhibitors on human KCNQ4 channels expressed in Xenopus laevis oocytes.
Methods:
Synthetic cRNA encoding human KCNQ4 channels was injected into Xenopus oocytes. We used a two-electrode voltage clamp to measure the ion currents in the oocytes.
Results:
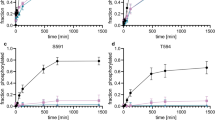
Wild-type KCNQ4 expressed in Xenopus oocytes showed the typical properties of slow activation kinetics and low threshold activation. The outward K+ current was almost completely blocked by a KCNQ4 blocker, linopirdine (0.25 mmol/L). BIMI (a PKC inhibitor) prevented the effects of PMA (a PKC activator) on the KCNQ4 current, indicating that PKC may be involved in the regulation of KCNQ4 expressed in the Xenopus oocyte system. Treatment with the ser/thr phosphatase inhibitors, cyclosporine (2 μmol/L), calyculin A (2 μmol/L) or okadaic acid (1 μmol/L), caused a significant positive shift in V1/2 and a decrease in the conductance of KCNQ4 channels. The V1/2 was shifted from −14.6±0.5 to −6.4±0.4 mV by cyclosporine, −18.8±0.5 to −9.2±0.4 mV by calyculin A, and −14.1±0.5 to −0.7±0.6 mV by okadaic acid. Moreover, the effects of these phosphatase inhibitors (okadaic acid or calyculin A) on the induction of a positive shift of V1/2 were augmented by further addition of PMA.
Conclusion:
These results indicate that ser/thr phosphatase inhibitors can induce a shift to more positive potentials of the activation curve of the KCNQ4 current. It is highly likely that the phosphatase functions to balance the phosphorylated state of substrate protein and thus has an important role in the regulation of human KCNQ4 channels expressed in Xenopus oocytes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA 2000; 97: 43338.
Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells is, mutated in dominant deafness. Cell 1999; 96: 437–46.
Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev 2003; 55: 583–6.
Marrion NV . Control of M-current. Annu Rev Physiol 1997; 59: 483–504.
Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D . Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J 2003; 22: 541221.
Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, et al. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 2003; 37: 963–75.
Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci 2003; 6: 564571.
Suh BC, Hille B . Does diacylglycerol regulate KCNQ channels? Pflugers Archive Eur J Physiol 2006; 453: 293–301.
Nakajo K, Kubo Y . Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes. J Physiol 2005; 569: 59–74.
Delmas P, Brown DA . Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005; 6: 85062.
Su CC, Li SY, Yang JJ, Su MC, Lin MJ . Studies of the effect of ionomycin on the KCNQ4 channel expressed in Xenopus oocytes. Biochem Biophys Res Commun 2006; 348: 295300.
Gamper N, Stockland JD, Shapiro MS . Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J Neurosci 2003; 23: 84–95.
Chambard JM, Ashmore JF . Regulation of the voltage-gated potassium channel KCNQ4 in the auditory pathway. Pfluger Arch Eur J Physiol 2005; 450: 34–44.
Surti TS, Huang L, Jan YN, Jan LY, Cooper EC . Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc Natl Acad Sci USA 2005; 102: 17828–33.
Schwake M, Jentsch TJ, Friedrich T . A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep 2003; 4: 76–81.
Howard RJ, Clark KA, Holton JM . Minor DL Jr . Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron 2007; 53: 66375.
Walker DH, DePaoli-Roach AA, Maller JL . Multiple roles for protein phosphatase 1 in regulating the Xenopus early embryonic cell cycle. Mol Biol Cell 1992; 3: 68798.
Lee TH . The role of protein phosphatase type-2A in the Xenopus cell cycle: initiation of the G2/M transition. Semin Cancer Biol 1995; 6: 2039.
Janssens V, Goris J . Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J 2001; 353: 41739.
Acknowledgements
Prof Thomas J JENTSCH is acknowledged for kindly supplying human KCNQ4 cDNA. These studies were supported by grants from the National Science Council, Taiwan (NSC 96-2320-B-040-013), CSMU-TSMH-097-007, and TCRD-TPE-95-39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, Tr., Chen, Ch., Huang, Sj. et al. Functional study of the effect of phosphatase inhibitors on KCNQ4 channels expressed in Xenopus oocytes. Acta Pharmacol Sin 30, 1220–1226 (2009). https://doi.org/10.1038/aps.2009.117
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.117
Keywords
This article is cited by
-
Action potential bursts in central snail neurons elicited by paeonol: roles of ionic currents
Acta Pharmacologica Sinica (2010)