Abstract
Aim:
MgFe2O4 magnetic nanoparticle composed of As2O3 (As2O3-MNPs) were prepared and their in vitro and in vivo characteristics were studied.
Methods:
The solvent-displacement method was applied for preparation of the nanoparticle using Poly-D,L-lactic-co-glycolic acid(PLGA). The characteristics studies of the products included magnetic response, morphology (transmission electron microscopy and scanning electron microscopy), entrapment efficiency, drug loading, particle sizes, zeta potential, in vitro drug release and tissue magnetic targeting. Nanoparticle cytotoxicity to Saos-2 cells was investigated using the MTT assay. To guide the external magnetic field in the liver, the concentration of As2O3 in the liver and kidney was measured using an atomic fluorescence spectrometer after injecting As2O3-MNPs into the caudal veins of mice.
Results:
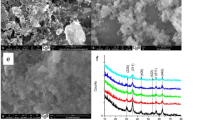
The As2O3-MNPs were approximately spherical. The average diameter, drug loading, entrapment efficiency and zeta potential of As2O3-MNPs were 109.9 nm, 10.08%, 82.16%, and −14.33 mV, respectively. The specific saturation magnetism was 8.65 emu/g. In vivo, the concentration of As2O3 in the liver was significantly higher than that in the non-magnetic group. While the concentration of As2O3 in the kidney was lower than that in the non-magnetic group. The Cmax in liver tissue in the magnetic group was 30.65 μg/g, which was 4.17 times the drug concentration in the same group in kidney tissue (7.35 μg/g) and 2.88 times the concentration of drug (10.66 μg/g) in the liver tissue of the non-magnetic group.
Conclusion:
The PLGA polymer-loaded magnetic nanoparticle composed of arsenic trioxide can be magnetically targeted well and applied in biomedicine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lew YS, Brown SL, Griffin RJ, Song CW, Kim JH . Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res 1999; 59: 6033–7.
Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, Rostami S, Jahani M, Hosseini R, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol 2006; 17: 131–4.
Slack JL, Waxman S, Tricot G, Tallman MS, Bloomfield CD . Advances in the management of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide. Oncologist 2002; 7: 1–13.
Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z . Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Phil Trans R Soc B 2007; 362: 959–71.
Zhu J, Chen Z, Lallemand-Breitenbach V, de Thé H . How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer 2002; 2: 1–9.
Gielen M, Tiekink ER . Metallotherapeutic drugs and metal-based diagnostic agents. Wiley; 2005. p 298.
Griffin RG, Williams BW, Park HJ, Song CW . Preferential action of arsenic trioxide in solid-tumor microenvironment enhances radiation therapy. Int J Radiat Oncol Biol Phys 2005; 61: 1516–22.
Zhou J, Meng R, Sui X, Meng L, Jia J, Yang B . Effects of administration styles of arsenic trioxide on intracellular arsenic concentration, cell differentiation and apoptosis. Haematologica 2005; 90: 1277–9.
Tun-Kyi A, Qin JZ, Oberholzer PA, Navarini AA, Hassel JC, Dummer R, et al. Arsenic trioxide down-regulates antiapoptotic genes and induces cell death in mycosis fungoides tumors in a mouse model. Ann Oncol 2008; 19: 1488–94.
Catarina PR, Ronald JN, António J R, Francisco V . Nanoencapsulation I . Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine 2006; 2: 8–21.
Du L, Chen JZ, Qi YT, Li D, Yuan CG, Lin MC, et al. Preparation and biomedical application of a non-polymer coated superparamagnetic nanoparticle. Int J Nanomed 2007; 2: 805–12.
Sunho P, Kimberly HS . Evaluation of hydrodynamic size and zeta-potential of surface-modified au nanoparticle-dna conjugates via ferguson analysis. J Phys Chem C 2008; 112: 7611–6.
Liu WY . Pharmaceutical analysis. Beijing: People's Medical Publishing House; 2007. 5th edition: p 287–336.
Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Carlos Palomino J . Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 2005; 55: 500–5.
Chou WC, Dang CV . Acute promyelocytic leukemia: recent advances in therapy and molecular basis of response to arsenic therapies. Curr Opin Hematol 2005; 12: 1–6.
Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S . Mechanisms of action of arsenic trioxide. Cancer Res 2002; 62: 3893–903.
Chun W, Phuong-Truc P . Polymers for viral gene delivery. Expert Opin Drug Deliv 2008; 5: 385–401.
Stevanovi M, Uskokovi D . Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci 2009; 5: 1–15.
Chen ZP, Zhang Y, Xu K, Xu RZ, Liu JW, Gu N . Stability of hydrophilic magnetic nanoparticles under biologically relevant conditions. J Nanosci Nanotechnol 2008; 8: 6260–5.
Yue PF, Yuan HL, Yang M, You RH, Cong LB, Zhu J, et al. Preparation, characterization, and pharmacokinetic evaluation of puerarin submicron emulsion. PDA J Pharm Sci Technol 2008; 62: 32–45.
Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY . A mechaninstic study of enhanced doxorubicin uptake and potention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticles system. J Pharmacol Exp Ther 2006; 317: 1372–81.
Acknowledgements
This project was supported by Science and technology key projects of Heilongjiang province (No GC08C421).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Gf., Li, Xh., Zhao, Z. et al. Preparation, characterization, in vivo and in vitro studies of arsenic trioxide Mg-Fe ferrite magnetic nanoparticles. Acta Pharmacol Sin 30, 1688–1693 (2009). https://doi.org/10.1038/aps.2009.158
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.158