Abstract
Aim:
It is unclear why α1D-adrenergic receptors (α1D-ARs) play a critical role in the mediation of peripheral vascular resistance and blood pressure in situ but function inefficiently when studied in vitro. The present study examined the causes for these inconsistencies in native α1-adrenergic functional performance between the vascular smooth muscle and myocytes.
Methods:
The α1-adrenergic mediated contraction, Ca2+ signaling and the subcellular receptor distribution were evaluated using the Fluo-4, BODIPY-FL prazosin and subtype-specific antibodies.
Results:
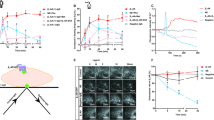
Rat aortic rings and freshly dissociated myocytes displayed contractile and increased intracellular Ca2+ responses to stimulation with phenylephrine (PE, 10 μmol), respectively. However, the PE-induced responses disappeared completely in cultured aortic myocytes, whereas PE-enhanced Ca2+ transients were seen in cultured rat cardiac myocytes. Further studies indicated that α1D-ARs, the major receptor subtype responsible for the α1-adrenergic regulation of aortic contraction, were distributed both intracellularly and at the cell membrane in freshly dispersed aortic myocytes, similar to the α1A-AR subcellular localization in the cultured cardiomyocytes. In the cultured aortic myocytes, however, in addition to a marked decrease in their protein expression relative to the aorta, most labeling signals for α1D-ARs were found in the cytoplasm. Importantly, treating the culture medium with charcoal/dextran caused the reappearance of α1D-ARs at the cell surface and a partial restoration of the Ca2+ signal response to PE in approximately 30% of the cultured cells.
Conclusion:
Reduction in α1D-AR total protein expression and disappearance from the cell surface contribute to the insensitivity of cultured vascular smooth muscle cells to α1-adrenergic receptor activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wu D, Katz A, Lee CH, Simon MI . Activation of phospholipase C by alpha1-adrenergic receptors is mediated by the subunits of Gq family. J Biol Chem 1992; 267: 25798–802.
Graham RM, Perez DM, Hwa J, Piascik MT . Alpha1-adrenergic receptor subtypes. Molecular structure, function and signaling. Circ Res 1996; 78: 737–49.
Michelotti GA, Price DT, Schwinn DA . Alpha1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther 2000; 88: 281–309.
Albert AP, Large WA . Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol 2006; 570 (Pt 1): 45–51.
Zhong H, Minneman KP . Alpha1-adrenoceptor subtypes. Eur J Pharmacol 1999; 375: 261–76.
Piascik MT, Perez DM . Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther 2001; 298: 403–10.
Milligan G . Exploring the dynamics of regulation of G protein-coupled receptors using green fluorescent protein. Br J Pharmacol 1999; 128: 501–10.
Chalothorn D, McCune DF, Edelmann SE, Garcia-Cazarin ML, Tsujimoto G, Piascik MT . Differences in the cellular localization and agonist-mediated internalization properties of the alpha1-adrenoceptor subtypes. Mol Pharmacol 2002; 61: 1008–16.
Theroux TL, Esbenshade TA, Peavy RD . Minneman KP . Coupling efficiencies of human alpha1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. J Pharmacol Exp Ther 1996; 50: 1376–87.
Uberti MA, Hall RA, Minneman KP . Subtype specific dimerization of alpha1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol Pharmacol 2003; 64: 1379–90.
Hein P, Michel MC . Signal transduction and regulation: Are all alpha1-adrenergic receptor subtypes created equal? Biochem Pharmacol 2007; 73: 1097–106.
Perez DM . Structure–function of alpha1-adrenergic receptors. Biochem Pharmacol 2007; 73: 1051–62.
Kenny BA, Chalmers DH, Philpott PC, Naylor AM . Characterization of an alpha1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br J Pharmacol 1995; 115: 981–6.
Gisbert R, Pérez-Vizcaino F, Cogolludo AL, Noguera MA, Ivorra MD, Tamargo J, et al. Cytosolic Ca2+ and phosphoinositide hydrolysis linked to constitutively active alpha1D-adrenoceptors in vascular smooth muscle. J Pharmacol Exp Therap 2003; 305: 1006–14.
Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 2002; 109: 765–75.
Hosoda C, Koshimizu T, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol 2005; 67: 912–22.
Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, et al. Subtype specific regulation of human vascular alpha1-adrenergic receptors by vessel bed and age. Circ 1999; 100: 2336–43.
Tanoue A, Koba M, Miyawaki S, Koshimizu T, Hosoda C, Oshikawa S, et al. Role of the alpha1D-adrenegric receptor in the development of salt-induced hypertension. Hypertension 2002; 40: 101–6.
Oliver E, Martí D, Montó F, Flacco N, Moreno L, Barettino DM, et al. The impact of alpha1-adrenoceptors up-regulation accompanied by the impairment of β-adrenergic vasodilatation in hypertension. J Pharmacol Exp Ther 2009; 328: 982–90.
Chaulet H, Lin F, Guo J, Owens WA, Michalicek J, Kesteven SH, et al. Sustained augmentation of cardiac alpha1A-adrenergic drive results in pathological remodeling with contractile dysfunction, progressive fibrosis and reactivation of matricellular protein genes. J Mol Cell Cardiol 2006; 40: 540–52.
Luo D, Yang D, Lan X, Li K, Li X, Chen J, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphoshate receptors in neonatal rat cardiomyocytes. Cell Calcium 2008; 43: 165–74.
Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB . Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem 2002; 277: 14266–73.
García KD, Shah T, García J . Immunolocalization of type 2 inositol 1,4,5-trisphosphate receptors in cardiac myocytes from newborn mice. Am J Physiol Cell Physiol 2004; 287: C1048–C57.
Autelitano DJ, Woodcock EA . Selective activation of alpha1A-adrenergic receptors in neonatal cardiac myocytes is sufficient to cause hypertrophy and differential regulation of alpha1-adrenergic receptor subtype mRNAs. J Mol Cell Cardiol 1998; 30: 1515–23.
Luo D, Nakazawa M, Ishibashi T, Kato K, Imai S . Putative, selective inhibitors of sarcoplasmic reticulum Ca2+-pump ATPase inhibit relaxation by nitroglycerin and atrial natriuretic factor of the rabbit aorta contracted by phenylephrine. J Pharmacol Exp Ther 1993; 265: 1187–92.
Zhou H, Iwasaki H, Nakamura T, Nakamura K, Maruyama T, Hamano S, et al. 2-Aminoethyl diphenylborinate analogues: Selective inhibition for store-operated Ca2+ entry. Biochem Biophys Res Commun 2007; 352: 277–82.
Rapps JA, Sturek M, Jones AW, Parker JL . Altered reactivity of coronary arteries located distal to a chronic coronary occlusion. Am J Physiol 1997; 273 (4 Pt 2): H1879–H87.
Villalba N, Stankevicius E, Garcia-Sacristán A, Simonsen U, Prieto D . Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am J Physiol Heart Circ Physiol 2007; 292: H1157–H69.
Erlinge D . Extracellular ATP: a central player in the regulation of vascular smooth muscle phenotype. Focus on “Dual role of PKA in phenotype modulation of vascular smooth muscle cells by extracellular ATP”. Am J Physiol Cell Physiol 2004, 287: C260–2.
Serir K, Hayoz S, Fanchaouy M, Beny JL, Bychkov R . A delayed ATP-elicited K+ current in freshly isolated smooth muscle cells from mouse aorta. Br J Pharmacol 2006; 147: 45–54.
Woodcock EA, Du XJ, Reichelt ME, Graham RM . Cardiac alpha1-adrenergic drive in pathological remodeling. Cardiovasc Res 2008; 77: 452–62.
Knowlton KU, Michel MC, Itani M, Shubeita HE, Ishihara K, Brown JH . The alpha1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem 1993; 268: 15374–80.
Luo D, Gao J, Fan L, Tang Y, Zhang Y, Han Q . Receptor subtype involved in alpha1-adrenergic receptor-mediated Ca2+ signaling in cardiomyocytes. Acta Pharmacol Sin 2007; 28: 921–1086.
Hanft G, Gross G . Subclassification of alpha1-adrenoceptor recognition sites by urapidil derivatives and other selective antagonists. Br J Pharmacol 1989; 97: 691–700.
Khattar SK, Bora RS, Priyadarsiny P, Gautam A, Gupta D, Tiwari A, et al. Molecular cloning, stable expression and cellular localization of human alpha1-adrenergic receptor subtypes: effect of charcoal/dextran treated serum on expression and localization of alpha1D-adrenergic receptor. Biotechnol Lett 2006; 28: 1731–9.
Mackenzie JF, Daly CJ, Pedian JI, Mcgrath JC . Quantitative imaging in live human cells reveals Intracellular alpha1-adrenoceptor ligand-binding sites. J Pharmacol Exp Ther 2000; 294: 434–43.
Woodsome TP, Polzin A, Kitazawa K, Eto M . Kitazawa T . Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci 2006; 119: 1769–80.
Koshimizu T, Tsujimoto G, Hirasawa A, Kitagawa Y, Tanoue A . Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovas Res 2004; 63: 662–72.
Lindquist DL, de Alarcon PA . Charcoal-dextran treatment of fetal bovine serum removes an inhibitor of human CFU-megakaryocytes. Exp Hematol 1987; 15: 234–38.
Tsang LL, Chan LN, Liu CQ, Chan HC . Effect of phenol red and steroid hormones on cystic fibrosis transmembrane conductance regulator in mouse endometrial epithelial cells. Cell Biol Int 2001; 25: 1021–4.
Hague C, Chen Z, Pupo AS, Schulte NA, Toews ML, Minneman KP . The N terminus of the human alpha1D-adrenergic receptor prevents cell surface expression. J Pharmacol Exp Ther 2004; 309: 388–97.
Lyssand JS, DeFino MC, Tang X, Hertz AL, Feller DB, Wacker JL, et al. Blood pressure is regulated by an alpha1D-adrenergic receptor/dystrophin signalosome. J Biol Chem 2008; 283: 18792–800.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation (No 30772574), the Beijing Municipal Project for Developing Advanced Human Resources for Higher Education, the Scientific Research Common Program of the Beijing Municipal Commission of Education (DL), and the Beijing Natural Science Foundation (No 7082018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, Ll., Ren, S., Zhou, H. et al. α1D-Adrenergic receptor insensitivity is associated with alterations in its expression and distribution in cultured vascular myocytes. Acta Pharmacol Sin 30, 1585–1593 (2009). https://doi.org/10.1038/aps.2009.160
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.160