Abstract
Aim:
To investigate the effect of ginsenoside Rg1 on the migration, adhesion, proliferation, and VEGF expression of endothelial progenitor cells (EPCs).
Methods:
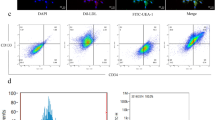
EPCs were isolated from human peripheral blood and incubated with different concentrations of ginsenoside Rg1 (0.1, 0.5, 1.0, and 5.0 μmol/L) and vehicle controls. EPC migration was detected with a modified Boyden chamber assay. EPC adhesion was determined by counting adherent cells on fibronectin-coated culture dishes. EPC proliferation was analyzed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In vitro vasculogenesis was assayed using an in vitro vasculogenesis detection kit. A VEGF-ELISA kit was used to measure the amount of VEGF protein in the cell culture medium.
Results:
Ginsenoside Rg1 promoted EPC adhesion, proliferation, migration and in vitro vasculogenesis in a dose- and time-dependent manner. Cell cycle analysis showed that 5.0 μmol/L of ginsenoside Rg1 significantly increased the EPC proliferative phase (S phase) and decreased the resting phase (G0/G1 phase). Ginsenoside Rg1 increased vascular endothelial growth factor production.
Conclusion:
The results indicate that ginsenoside Rg1 promotes proliferation, migration, adhesion and in vitro vasculogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7.
Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006; 152: 190–5.
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999–1007.
Le Ricousse-Roussanne S, Barateaua V, Contreres JO, Boval B, Kraus-Berthier L, Tobelem G . Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res 2004; 62: 176–84.
Gillis CN . Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 1997; 54: 1–8.
Radad K, Gille G, Moldzio R, Saito H, Rausch WD . Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res 2004; 1021: 41–53.
Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC, Lai PH, et al. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release 2007; 120: 27–34.
Cheng Y, Shen LH, Zhang JT . Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin 2005; 26: 143–9.
Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 2003; 107: 3059–65.
Lau WS, Chan RY, Guo DA, Wong MS . Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem Mol Biol 2008; 108: 64–71.
Saw Imanishi T, Hano T, Matsuo Y, Nishio I . Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharmacol Physiol 2003; 30: 665–70.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600.
Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000; 97: 3422–7.
Chen JZ, Zhu JH, Wang XX, Zhu JH, Xie XD, Sun J, et al. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol 2004; 36: 233–9.
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89: E1–E7.
Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002; 105: 3017–24.
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002; 106: 2781–6.
Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM . Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 2002; 109: 337–46.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–6.
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103: 634–7.
Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization. Exp Hematol 2002; 30: 967–72.
Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, et al. Neoendothelialization after peripheral blood stem cell transplantation in humans. A case report of a Tokaimura nuclear accident victim. Cardiovasc Res 2003; 58: 487–92.
George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, et al. Number and adhesive properties of circulating endothelial progenitor cells in patients with instent restenosis. Arterioscler Thromb Vasc Biol 2003; 23: e57–60.
Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 2003; 42: 2073–80.
Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004; 24: 1442–7.
Imanishi T, Moriwaki C, Hano T, Nishio I . Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens 2005; 23: 1831–7.
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J Clin Invest 2001; 108: 391–7.
Spyridopoulos I, Haendeler J, Urbich C, Brummendorf TH, Oh H, Schneider MD, et al. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation 2004; 110: 3136–42.
Levy BI . Beneficial effects of circulating progenitor endothelial cells activated by angiotensin receptor antagonists. Hypertension 2005; 45: 491–2.
Blázquez C, González-Feria L, Alvarez L, Haro A, Casanova ML, Guzmán M . Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res 2004; 64: 5617–23.
Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25: 581–611.
Leung KW, Pon YL, Wong RN, Wong AS . Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem 2006; 281: 36280–8.
Lü JP, Ma ZC, Yang J, Huang J, Wang SR, Wang SQ . Ginsenoside Rg1-induced alterations in gene expression in TNF-alpha stimulated endothelial cells. Chin Med J (Engl) 2004; 117: 871–6.
Yang M, Chen GL, Chen C, Zhang Y, Yan CY . Therapeutic effects of ginsenoside Rg1 on rat with myocardial infarction. Chin J Integr Med Cardio/Cerebrovasc Dis 2007; 5: 1075–7.
Xu Q . Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol 2004; 165: 1–10.
Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000; 105: 1527–36.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–6.
Yu LC, Chen SC, Chang WC, Huang YC, Lin KM, Lai PH, et al. Stability of angiogenic agents, ginsenoside Rg1 and Re, isolated from Panax ginseng: in vitro and in vivo studies. Int J Pharm 2007; 328: 168–76.
Lu XZ, Wang JH, Wu X, Zhou L, Wang L, Zhang XW, et al. Ginsenoside Rg1 promotes bone marrow stromal cells proliferation via the activation of the estrogen receptor-mediated signaling pathway. Acta Pharmacol Sin 2008; 29: 1209–14.
Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK . Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol 1997; 133: 135–40.
Chung E, Lee KY, Lee YH, Lee YJ, Lee SK . Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids 1998; 63: 421–4.
Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN . Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett 2006; 580: 3211–6.
Liang HC, Chen CT, Chang Y, Huang YC, Chen SC, Sung HW . Loading of a novel angiogenic agent, ginsenoside Rg1 in an acellular biological tissue for tissue regeneration. Tissue Eng 2005; 11: 835–46.
Acknowledgements
The authors gratefully acknowledge helpful support from Prof Chang-fen XU. We also thank Zhu-ping TANG and Bo FENG for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Aw., Wang, Xb., Lu, Fx. et al. Ginsenoside Rg1 promotes endothelial progenitor cell migration and proliferation. Acta Pharmacol Sin 30, 299–306 (2009). https://doi.org/10.1038/aps.2009.6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.6
Keywords
This article is cited by
-
Ginsenoside Rg1 attenuates the NASH phenotype by regulating the miR-375-3p/ATG2B/PTEN-AKT axis to mediate autophagy and pyroptosis
Lipids in Health and Disease (2023)
-
Screening ginseng saponins in progenitor cells identifies 20(R)-ginsenoside Rh2 as an enhancer of skeletal and cardiac muscle regeneration
Scientific Reports (2020)
-
In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231
BMC Complementary and Alternative Medicine (2018)
-
An in vivo study of hypoxia-inducible factor-1α signaling in ginsenoside Rg1-mediated brain repair after hypoxia/ischemia brain injury
Pediatric Research (2017)
-
Ginsenoside Rb1 prevents homocysteine-induced EPC dysfunction via VEGF/p38MAPK and SDF-1/CXCR4 activation
Scientific Reports (2017)