Abstract
Aim:
To investigate the mechanism of the bone-forming effects of intermittent parathyroid hormone (PTH) administration and to search for novel molecules of bone anabolism via the PTH signaling pathway.
Methods:
Primary cultures of rat osteoblasts (ROBs) were divided into an intermittent PTH-treated group (Itm) and a control group (Ctr). Imitating the pharmacokinetics of intermittent PTH administration in vivo, the ROBs in the Itm group were exposed to PTH for 6 h in a 24-h incubation cycle, and the ROBs in the Ctr group were exposed to vehicle for the entire incubation cycle. The cells were collected at 6 h and 24 h of the final cycle, and the proteins in the Itm and Ctr groups were analyzed by two-dimensional electrophoresis (2-DE) coupled with peptide mass fingerprinting and matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) to detect proteins that were differentially expressed. The proteins with the most significant changes in vitro were validated by immunohistochemistry (IHC) in a rat model.
Results:
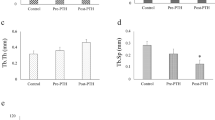
The proteomics analysis indicated that a total of 26 proteins were up- or down-regulated in the Itm group compared with the Ctr group at 6 h and 24 h; among these, 15 proteins were successfully identified. These proteins mainly belong to the cytoskeleton and molecular chaperone protein families, and most of these have anti-apoptotic effects in various cells. Rho GDP-dissociation inhibitor α (RhoGDIα) and vimentin were the most significantly changed proteins. Further studies by IHC showed that the expression of RhoGDIα in ROBs was significantly higher in PTH-treated sham-operated rats than in vehicle-treated sham-operated rats, but the difference was not significant between PTH-treated and vehicle-treated OVX rats. Vimentin expression was not changed in either PTH-treated sham-operated rats or PTH-treated OVX rats.
Conclusion:
Our research suggests that intermittent PTH treatment induces changes in expression of many proteins in ROBs in vitro, and it results in RhoGDIα up-regulation in ROBs both in vitro and in vivo when estrogen is present. This up-regulation of RhoGDIα may be one of the mechanisms underlying the synergistic bone-forming effect of PTH and estrogen.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, et al. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 2003; 88: 1150–6.
Gabet Y, Kohavi D, Muller R, Chorev M, Bab I . Intermittently administered parathyroid hormone (1–34) reverses bone loss and structural impairment in orchiectomized adult rats. Osteoporos Int 2005; 16: 1436–43.
Sato M, Westmore M, Ma YL, Schmidt A, Zeng QQ, Glass EV, et al. Teriparatide [PTH(1–34)] strengthens the proximal femur of ovariectomized nonhuman primates despite increasing porosity. J Bone Miner Res 2004; 19: 623–9.
Hock JM, Gera I . Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 1992; 7: 65–72.
Qin L, Qiu P, Wang LQ, Li X, Swarthout JH, Soteropoulou P, et al. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol chem 2003; 278: 19723–31.
Gygi SP, Rochon Y, Robert Franza B, Aebersold R . Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999; 19: 1720–30.
Guo QC, Shen JN, Jin S, Wang J, Huang G, Zhang LJ, et al. Comparative proteomic analysis of human osteosarcoma and SV40-immortalized normal osteoblastic cell lines. Acta Pharmacol Sin 2007; 28: 850–8.
Zhang KQ, Chen JW, Wang ML, Wang C, Li G, Zheng Z, et al. The expression of insulin-like growth factor-I mRNA and polypeptide in rat osteoblasts with exposure to parathyroid hormone. Chin Med J (Engl) 2003; 116: 1916–22.
Jiang LL, Jiang H, Liu CP, Zhang KQ . The secretome profile in rat osteoblasts intermittently exposed to parathyroid hormone. Chin J Osteoporosis Bone Miner Res 2008; 1: 53–8.
Mcsheehy PM, Chambers TJ . Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. J Endocrinol 1986; 118: 824–8.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1976; 72: 248–54.
Schiller PC, D'Ippolito G, Roos BA, Howard GA . Anabolic or catabolic responses of MC3T3-E1 osteoblastic cells to parathyroid hormone depend on time and duration of treatment. J Bone Miner Res 1999; 14: 1504–12.
Kroll MH . Parathyroid hormone temporal effects on bone formation and resorption. Bull Math Biol 2000; 62: 163–88.
Gourlay CW, Ayscough KR . The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol 2005; 6: 583–9.
Morishima N . Changes in unclear morphology during apoptosis correlate with vimentin cleavage by different caspases located either upstream or downstream of Bcl-2 action. Genes to Cell 1999; 4: 401–14.
Tanaka H, Moriwake T, Matsuoka Y, Nakamura T, Seino Y . Potential role of rhIGF-I/IGFBP-3 in maintaining skeletal mass in space. Bone 1998; 22: 145S–147S.
Silha JV, Mishra S, Rosen CJ, Beamer WG, Turner RT, Powell DR, et al. Perturbations in bone formation and resorption in insulin-like growth factor binding protein-3 transgenic mice. J Bone Miner Res 2003; 18: 1834–41.
Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, et al. Molcular cloning characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 1990; 5: 1321–8.
Leonard D, Hart MJ, Platko JV, Eva A, Henzel W, Evans T, et al. The identification and characterization of a GDP-dissociation inhibitor (GDI) for the CDC42Hs protein. J Biol Chem 1992; 267: 22860–8.
Hall A . Rho GTPase and the actin cytoskeleton. Science 1998; 279: 509–14.
Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking RhoGDI. Oncogene 1999; 18: 5373–80.
Jones MB, Krutzsch H, Shu H, Zhao YM, Liotta LA, Kohn EC, et al. Proteomic analysis and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics 2002; 2: 76–84.
Fritz G, Just I, Kaina B . Rho GTPases are over-expressed in human tumors. Int J Cancer 1999; 81: 682–7.
Su LF, Knoblauch R, Garabedian MJ . Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem 2001; 276: 3231–7.
Smith EP, Boyd J, Frank GR, Takabashi H, Cohen RM, Speeker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994; 331: 1056–61.
Korach KS . Insights from the study of animals lacking functional estrogen receptor. Science 1994; 266: 1524–7.
Lee KCL, Jessop H, Suswillo R, Zaman G, Lanyon LE . The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of estrogen receptor-α and –β. J Endocrinol 2004; 182: 193–201.
Mcdougall KE, Perry MJ, Gibson RL, Colley SM, Korach KS, Tobias JH . Estrogen receptor-α dependency of estrogen's stimulatory action on cancellous bone formation in male mice. J Endocrinol 2003; 144: 1994–9.
Shen V, Birchman R, Xu R, Otter M, Wu D, Lindsay R, Dempster DW . Effects of reciprocal treatment with estrogen and estrogen plus parathyroid hormone on bone structure and strength in ovariectomized rats. J Clin Invest 1995; 96: 2331–8.
Bradbeer JN, Arlot ME, Meunier PJ, Reeve J . Treatment of osteoporosis with parathyroid peptide (hPTH 1–34) and oestrogen: increase in volumetric density of iliac cancellous bone may depend on reduced trabecular spacing as well as increased thickness of packets of newly formed bone. J Clin Endocrinol 1992; 37: 282–9.
Zhang BL, Zhang YQ, Dagher MC, Shacter E . Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res 2005; 65: 6054–62.
Acknowledgements
This project was supported by the Research Foundation for the Returned Overseas Scholars of the Ministry of Education of China and of the Ministry of Personnel of China. We thank the Central Laboratory of Nanjing Medical University for technical support for the MS/MS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Zf., Jiang, H., Ye, Zq. et al. Expression of Rho GDIα in rat osteoblasts intermittently exposed to parathyroid hormone in vitro and in vivo. Acta Pharmacol Sin 30, 1001–1007 (2009). https://doi.org/10.1038/aps.2009.60
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.60