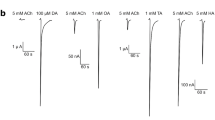
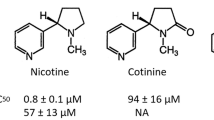
Abstract
Neuromodulator action has received increasing attention in theoretical neuroscience. Yet models involving both neuronal populations dynamics at the circuit level and detailed receptor properties are only now being developed. Here we review recent computational approaches to neuromodulation, focusing specifically on acetylcholine (ACh) and nicotine. We discuss illustrative examples of models ranging from functional top-down to neurodynamical bottom-up. In the top-down approach, a computational theory views ACh as encoding the uncertainty expected in an environment. A different line of models accounts for neural population dynamics treating ACh as toggling neuronal networks between read-in of information and recall of memory. Building on the neurodynamics idea we discuss two models of nicotine's action with increasing degree of biological realism. Both consider explicitly receptor-level mechanisms but with different scales of detail. The first is a large-scale model of nicotine-dependent modulation of dopaminergic signaling that is capable of simulating nicotine self-administration. The second is a novel approach where circuit-level neurodynamics of the ventral tegmental area (VTA) are combined with explicit models of the dynamics of specific nicotinic ACh receptor subtypes. We show how the model is constructed based on local anatomy, electrophysiology and receptor properties and provide an illustration of its potential. In particular, we show how the model can shed light on the specific mechanisms by which nicotine controls dopaminergic neurotransmission in the VTA. This model serves us to conclude that detailed accounts for neuromodulator action at the basis of behavioral and cognitive models are crucial to understand how neuromodulators mediate their functional properties.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fellous JM, Linster C . Computational models of neuromodulation. Neural Comput 1998; 10: 771–805.
Dayan P, Abbott LF . Theoretical neuroscience-computational and mathematical modeling of neural systems. Cambridge (MA): MIT Press; 2001.
Koch C, Segev I, editors. Methods in neuronal modeling: from ions to networks. 2nd edition. Cambridge (MA): MIT Press; 1998.
Katz PS . Beyond neurotransmission: Neuromodulation and its importance for information processing. Oxford: Oxford University Press; 1999.
Sutton RS, Barto AG . Reinforcement learning: an introduction. IEEE Trans Neural Netw 1998; 9: 1054.
Mitchell TM . Machine Learning. McGraw Hill; 1997.
Hasselmo ME . The role of acetylcholine in learning and memory. Curr Opin Neurobiol 2006; 16: 710–5.
Yu AJ, Dayan P . Acetylcholine in cortical inference. Neural Netw 2002; 15: 719–30.
Yu AJ, Dayan P . Uncertainty, neuromodulation, and attention. Neuron 2005; 46: 681–92.
Durstewitz D, Seamans JK . Beyond bistability: biophysics and temporal dynamics of working memory. Neuroscience 2006; 139: 119–33.
Durstewitz D, Seamans JK . The computational role of dopamine D1 receptors in working memory. Neural Netw 2002; 15: 561–72.
Phillips JM, McAlonan K, Robb WG, Brown VJ . Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berl) 2000; 150: 112–6.
Hasselmo ME . Expecting the unexpected: modeling of neuromodulation. Neuron 2005; 46: 526–8.
Montague PR, Dayan P, Sejnowski TJ . A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 1996; 16: 1936–47.
Compte A, Wang XJ . Tuning curve shift by attention modulation in cortical neurons: a computational study of its mechanisms. Cereb Cortex 2006; 16: 761–78.
Hasselmo ME, Anderson BP, Bower JM . Cholinergic modulation of cortical associative memory function. J Neurophysiol 1992; 67: 1230–46.
Deco G, Rolls ET . Attention, short-term memory, and action selection: a unifying theory. Prog Neurobiol 2005; 76: 236–56.
Gil Z, Connors BW, Amitai Y . Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 1997; 19: 679–86.
Linster C, Cleland TA . Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw 2002; 15: 709–17.
Linster C, Hasselmo ME . Neuromodulation and the functional dynamics of piriform cortex. Chem Senses 2001; 26: 585–94.
Hasselmo ME, McGaughy J . High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res 2004; 145: 207–31.
Hertz J, Krogh A, Palmer RG . Introduction to the theory of neural computation. Redwood City (CA): Addison Wesley; 1991.
Hasselmo . Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci 1999; 3: 351–9.
Patil MM, Hasselmo ME . Modulation of inhibitory synaptic potentials in the piriform cortex. J Neurophysiol 1999; 81: 2103–18.
Myers CS, Taylor RC, Moolchan ET, Heishman SJ . Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology 2008; 33: 588–98.
Gutkin BS, Dehaene S, Changeux JP . A neurocomputational hypothesis for nicotine addiction. Proc Natl Acad Sci USA 2006; 103: 1106–11.
Corrigall WA, Coen KM . Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989; 99: 473–8.
David V, Besson M, Changeux JP, Granon S, Cazala P . Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 2006; 50: 1030–40.
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. Nature 1998; 391: 173–7.
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 2003; 23: 7820–9.
Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Léna C, et al. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Brain Res Rev 1998; 26: 198–216.
Dani JA, Heinemann S . Molecular and cellular aspects of nicotine abuse. Neuron 1996; 16: 905–8.
Usher M, McClelland JL . The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev 2001; 108: 550–92.
Cho RY, Nystrom LE, Brown ET, Jones AD, Braver TS, Holmes PJ, et al. Mechanisms underlying dependencies of performance on stimulus history in a two-alternative forced-choice task. Cogn Affect Behav Neurosci 2002; 2: 283–99.
Beiser DG, Hua SE, Houk JC . Network models of the basal ganglia. Curr Opin Neurobiol 1997; 7: 185–90.
Dehaene S, Changeux JP . Reward-dependent learning in neuronal networks for planning and decision making. Prog Brain Res 2000; 126: 217–29.
DiChiara G . Drug addiction as a dopamine-dependent associative learning disorder. Eur J Pharmacol 1999; 375: 13–30.
Rahman S, Zhang J, Engleman EA, Corrigall WA . Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience 2004; 129: 415–24.
Granon S, Faure P, Changeux JP . Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci USA 2002; 100: 9596–601.
Karlin A . Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 2002; 3: 102–14.
Edelstein SJ, Schaad O, Henry E, Bertrand D, Changeux JP . A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol Cybern 1996; 75: 361–79.
Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA . Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 1997; 17: 5747–59.
Monond J, Wyman J, Changeux JP . On the nature of allosteric transitions: a plausible model. J Mol Biol 1965; 12: 88–118.
Castillo JD, Katz B . Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci 1957; 146: 369–81.
Katz B, Thesleff S . A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 1957; 138: 63–80.
Colquhoun D, Sakmann B . Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol 1985; 369: 501–57.
Franke C, Parnas H, Hovav G, Dudel J . A molecular scheme for the reaction between acetylcholine and nicotinic channels. Biophys J 1993; 64: 339–56.
Heidmann T, Changeux JP . Interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata in the millisecond time range: resolution of an “intermediate” conformational transition and evidence for positive cooperative effects. Biochem Biophys Res Commun 1980; 97: 889–96.
Neubig RR, Cohen JB . Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry 1980; 19: 2770–9.
Auerbach A, Akk G . Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol 1998; 112: 181–97.
Prince RJ, Sine SM . Acetylcholine and epibatidine binding to muscle acetylcholine receptors distinguish between concerted and uncoupled models. J Biol Chem 1999; 274: 19623–9.
Reitstetter R, Lukas RJ, Gruener R . Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 1999; 289: 656–60.
Kalivas PW, Churchill L, Klitenick MA . GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 1993; 57:1047–60.
Christie MJ, Bridge S, James LB, Beart PM . Excitotoxin lesions suggest an aspartatergic projection from rat medial prefrontal cortex to ventral tegmental area. Brain Res 1985; 333: 169–72.
Cornwall J, Cooper JD, Phillipson OT . Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull 1990; 25: 271–84.
Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK . Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 1995; 15: 5859–69.
Johnson SW, North RA . Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 1992; 450: 455–68.
Mansvelder HD, Rover MD, McGehee DS, Brussaard AB . Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol 2003; 480: 117–23.
Mansvelder HD, McGehee DS . Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 2000; 27: 349–57.
Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M . Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 2007; 74: 1102–11.
Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP . Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 2001; 21: 1452–63.
Corrigall WA, Coen KM, Adamson KL . Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 1994; 653: 278–84.
Dani JA, Ji D, Zhou FM . Synaptic plasticity and nicotine addiction. Neuron 2001; 31: 349–52.
Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED . Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend 1993; 33: 23–9.
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA . Nicotine activates and desensitizes midbrain dopamine neurons. Nature 1997; 390: 401–4.
Wilson HR, Cowan JD . Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J 1972; 12: 1–24.
Hansel D, Sompolinsky H . Modeling Feature Selectivity in Local Cortical Circuits. In Methods in Neuronal Modeling: From Synapse to Networks. Koch C, Segev I, editors. Cambridge (MA): MIT Press; 1998.
Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J . Human alpha7 acetylcholine receptor: cloning of the alpha7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha7 homomers expressed in Xenopus oocytes. Mol Pharmacol 1994; 45: 546–54.
Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, et al. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol 1995; 48: 774–82.
Buisson B, Bertrand D . Chronic exposure to nicotine upregulates the human (alpha)4(beta)2 nicotinic acetylcholine receptor function. J Neurosci 2001; 21:1819–29.
Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, Krishnan C, et al. Characterization of human (alpha)4(beta)2-nicotinic acetylcholine receptors stably and heterologously expressed in native nicotinic receptor-null SH-EP1 human epithelial cells. Mol Pharmacol 2003; 64: 1283–94.
Papke RL . Estimation of both the potency and efficacy of alpha7 nAChR agonists from single-concentration responses. Life Sci 2006; 78: 2812–9.
Schultz W . Predictive reward signal of dopamine neurons. J Neurophysiol 1998; 80: 1–27.
Matsumoto M, Hikosaka O . Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007; 447: 1111–5.
Acknowledgements
This work is supported by CNRS, Collège de France, IST European consortium project BACS FP6-IST-027140 (MG and BG), École des Neurosciences de Paris Île-de-France (MG), and the Marie Curie Team of Excellence Grant BIND MECT-CT-20095-024831 (BG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graupner, M., Gutkin, B. Modeling nicotinic neuromodulation from global functional and network levels to nAChR based mechanisms. Acta Pharmacol Sin 30, 681–693 (2009). https://doi.org/10.1038/aps.2009.87
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2009.87
Keywords
This article is cited by
-
Simulating Small Neural Circuits with a Discrete Computational Model
Biological Cybernetics (2020)
-
In silico pharmacology: drug design and discovery’s gate to the future
In Silico Pharmacology (2013)
-
Modulation of dopamine release by α7-type nicotinic acetylcholine receptors
BMC Neuroscience (2013)
-
Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement
Molecular Psychiatry (2013)