Abstract
Aim:
To evaluate the role of glycogen synthase kinase-3β (GSK-3β) in the induced differentiation of human glioblastoma cells.
Methods:
Cell proliferation was determined by bromodeoxyuridine (BrdU) incorporation assay. The protein level of p-GSK-3β, GSK-3β, glial fibrillary acidic protein (GFAP) and proliferating cell nuclear antigen (PCNA) were determined using Western blots. The overexpression of mutant GSK-3β was analyzed by immunocytochemistry.
Results:
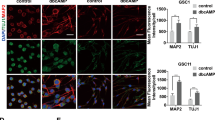
The biotoxin cholera toxin is capable of inducing differentiation of U87-MG human glioblastoma cells, which is characterized by morphological changes to astrocytic phenotype, increase in differentiation marker protein GFAP and decrease in proliferation. GSK-3β activation is induced during this differentiation. Small interfering RNA against GSK-3β suppresses the induced-differentiation in U87-MG cells. Conversely, overexpression of a constitutively active form of human GSK-3β (pcDNA3-GSK-3β-S9A) mutant leads to differentiation of U87-MG cells.
Conclusion:
Our findings suggest that GSK-3β plays an important role in astrocytic differentiation of human glioblastoma cells and may be a novel therapeutic target in the malignant tumor.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
DeAngelis LM . Brain tumors. N Engl J Med 2001; 344: 114–23.
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev 2001; 15: 1311–33.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–96.
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988; 72: 567–72.
Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB . Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther 2001; 90: 105–56.
Li Y, Yin W, Wang X, Zhu W, Huang Y, Yan G . Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc Natl Acad Sci USA 2007; 104: 13438–43.
Ougolkov AV, Billadeau DD . Targeting GSK-3: a promising approach for cancer therapy? Future Oncol 2006; 2: 91–100.
Woodgett JR . cDNA cloning and properties of glycogen synthase kinase-3. Methods Enzymol 1991; 200: 564–77.
Jope RS, Johnson GV . The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 2004; 29: 95–102.
Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung MC, et al. GSK-3 beta targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell 2008; 13: 36–47.
Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 2005; 19: 159–70.
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res 2006; 66: 11462–70.
Roymans D, Vissenberg K, De Jonghe C, Grobben B, Claes P, Verbelen JP, et al. Phosphatidylinositol 3-kinase activity is required for the expression of glial fibrillary acidic protein upon cAMP-dependent induction of differentiation in rat C6 glioma. J Neurochem 2001; 76: 610–8.
Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J . Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell 1994; 79: 1233–43.
Scott RE . Differentiation, differentiation/gene therapy and cancer. Pharmacol Ther 1997; 73: 51–65.
Sell S . Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol 2004; 51: 1–28.
Takanaga H, Yoshitake T, Hara S, Yamasaki C, Kunimoto M . cAMP-induced astrocytic differentiation of C6 glioma cells is mediated by autocrine interleukin-6. J Biol Chem 2004; 279: 15441–7.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116: 1175–86.
Manoukian AS, Woodgett JR . Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res 2002; 84: 203–29.
Polakis P . The oncogenic activation of beta-catenin. Curr Opin Genet Dev 1999; 9: 15–21.
Liao X, Zhang L, Thrasher JB, Du J, Li B . Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol Cancer Ther 2003; 2: 1215–22.
Mazor M, Kawano Y, Zhu H, Waxman J, Kypta RM . Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene 2004; 23: 7882–92.
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR . Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000; 406: 86–90.
Schwabe RF, Brenner DA . Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol 2002; 283: G204–11.
la Sala A, He J, Laricchia-Robbio L, Gorini S, Iwasaki A, Braun M, et al. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J Exp Med 2009; 206: 1227–35.
Kimura M, Hidari KI, Suzuki T, Miyamoto D, Suzuki Y . Engagement of endogenous ganglioside GM1a induces tyrosine phosphorylation involved in neuron-like differentiation of PC12 cells. Glycobiology 2001; 11: 335–43.
Masco D, Van de Walle M, Spiegel S . Interaction of ganglioside GM1 with the B subunit of cholera toxin modulates growth and differentiation of neuroblastoma N18 cells. J Neurosci 1991; 11: 2443–52.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No 30830111 and 30801408), National Natural Science Foundation of Guangdong Province (No 8451008901000297), Chinese Postdoctoral Science Foundation (No 20080430801 and 200801266), Special Foundation, Chinese Postdoctoral Science (No 200801266) and Guangzhou Scientific and Technological Programs (No 2008Z1-E561).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Lu, Hm., Li, G. et al. Glycogen synthase kinase-3β regulates astrocytic differentiation of U87-MG human glioblastoma cells. Acta Pharmacol Sin 31, 355–360 (2010). https://doi.org/10.1038/aps.2010.10
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.10
Keywords
This article is cited by
-
Oil source and migration process in oblique transfer zone of Fushan Sag, northern South China Sea
Journal of Central South University (2016)
-
Tight regulation between cell survival and programmed cell death in GBM stem-like cells by EGFR/GSK3b/PP2A signaling
Journal of Neuro-Oncology (2015)
-
Transtensional tectonism and its effects on the distribution of sandbodies in the Paleogene Baiyun Sag, Pearl River Mouth Basin, China
Marine Geophysical Research (2013)
-
Wnt/beta-Catenin Signaling in Glioma
Journal of Neuroimmune Pharmacology (2012)