Abstract
Aim:
To investigate the population pharmacokinetics of recombinant human tumor necrosis factor receptor-Fc fusion protein (rhTNFR-Fc) administered via subcutaneous (SC) injection in healthy Chinese volunteers and in Chinese patients with ankylosing spondylitis (AS).
Methods:
Thirty-two healthy volunteers were randomly assigned to receive a single SC injection of 12.5, 25, 37.5, or 50 mg of rhTNFR-Fc. Twenty male patients with moderate AS were randomly assigned to receive seven consecutive SC injections of rhTNFR-Fc at either 25 mg twice a week (BIW) or 50 mg once a week (QW). Population pharmacokinetic (PK) analysis was applied to obtain PK parameters of rhTNFR-Fc by the NONMEM method.
Results:
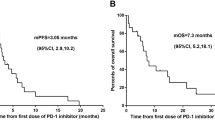
The data were best described by a one-compartment model with lag time. We found that gender had a significant effect on the apparent clearance (CL/F), with the male CL/F ratio being only 0.665 times the female ratio; the absorption coefficient (F) of multiple dosages of rhTNFR-Fc was only 0.674 times that of a single dosage. The outcome parameters were CL/F (female: 0.168 L/h, male: 0.110 L/h), the apparent volume of distribution (Vd/F: 15.5 L), the absorption rate constant (Ka) (single dosage: 0.0605 h−1, multiple dosage: 0.0408 h−1), and the lag time (Tlag: 1.03 h). The inter-individual variability in the CL/F, Vd/F, Ka, and Tlag were 33.3%, 42.7%, 55.6%, and 81.8%, respectively.
Conclusion:
Chinese females have a higher CL/F than Chinese males, and multiple dosings can significantly decrease the absorption of rhTNFR-Fc (SC). The population PK parameters of rhTNFR-Fc in healthy Chinese volunteers and patients with AS were similar to those reported for subjects in published American studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ng SC, Liao Z, Yu DT, Chan ES, Zhao L, Gu J . Epidemiology of spondyloarthritis in the People's Republic of China: review of the literature and commentary. Semin Arthritis Rheum 2007; 37: 39–47.
Gran JT, Skomsvoll JF . The outcome of ankylosing spondylitis: a study of 100 patients. Br J Rheumatol 1997; 36: 766–71.
Khalessi AA, Oh BC, Wang MY . Medical management of ankylosing spondylitis. Neurosurg Focus 2008; 24: E4.
Kalden JR . Emerging role of anti-tumor necrosis factor therapy in rheumatic diseases. Arthritis Res 2002; 4: S34–40.
Camussi G, Albano E, Tetta C, Bussolino F . The molecular action of tumor necrosis factor-alpha. Eur J Biochem 1991; 202: 3–14.
Crew MD, Effros RB, Walford RL, Zeller E, Cheroutre H, Brahn E . Transgenic mice expressing a truncated Peromyscus leucopus TNF-alpha gene manifest an arthritis resembling ankylosing spondylitis. J Interferon Cytokine Res 1998; 18: 219–25.
Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R . Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol 2007; 26: 211–5.
Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 1995; 38: 499–505.
Francois RJ, Neure L, Sieper J, Braun J . Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis 2006; 65: 713–20.
Amgen-Wyeth Enbrel Prescribing Information. United States 2009.
Braun J, Sieper J, Breban M, Collantes-Estevez E, Davis J, Inman R, et al. Anti-tumour necrosis factor alpha therapy for ankylosing spondylitis: international experience. Ann Rheum Dis 2002; 61: iii51–60.
Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003; 48: 1667–75.
Davis JC, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003; 48: 3230–6.
Baraliakos X, Davis J, Tsuji W, Braun J . Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept. Arthritis Rheum 2005; 52: 1216–23.
Brandt J, Listing J, Haibel H, Sorensen H, Schwebig A, Rudwaleit M, et al. Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford) 2005; 44: 342–8.
Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME . The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother 2000; 34: 161–4.
Zhou H . Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol 2005; 45: 490–7.
Lee H, Kimko HC, Rogge M, Wang D, Nestorov I, Peck CC . Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin Pharmacol Ther 2003; 73: 348–65.
Yim DS, Zhou H, Buckwalter M, Nestorov I, Peck CC, Lee H . Population pharmacokinetic analysis and simulation of the time-concentration profile of etanercept in pediatric patients with juvenile rheumatoid arthritis. J Clin Pharmacol 2005; 45: 246–56.
Kawai S, Sekino H, Yamashita N, Tsuchiwata S, Liu H, Korth-Bradley JM . The comparability of etanercept pharmacokinetics in healthy Japanese and American subjects. J Clin Pharmacol 2006; 46: 418–23.
Acknowledgements
This work was financially supported by Celgen Bio-Pharmaceutical Co Ltd (Shanghai, China). The authors would like to thank Prof Rui WANG for direction of the clinical work. We are also thankful to Prof Feng HUANG, Prof Ze-ling CAI, Mr Xiao-hu DENG, and Ms Chun-hua YANG from General Hospital of PLA for their technical assistance. Thanks to our families for their understanding and support in our study, work, and life.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fang, Y., Li, Lj., Wang, R. et al. Population pharmacokinetics of rhTNFR-Fc in healthy Chinese volunteers and in Chinese patients with Ankylosing spondylitis. Acta Pharmacol Sin 31, 1500–1507 (2010). https://doi.org/10.1038/aps.2010.113
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/aps.2010.113
Keywords
This article is cited by
-
Pharmacokinetics and immunogenicity of T0001, a newly developed anti-TNFα fusion protein, in healthy volunteers
European Journal of Clinical Pharmacology (2017)